PETAH TIKVA (dpa-AFX) - Teva Pharmaceuticals, the U.S. affiliate of Teva Pharmaceutical Industries Ltd. (TEVA), in collaboration with Medincell (MEDCL.PA), announced that the U.S. Food and Drug Administration has approved UZEDY (risperidone) extended-release injectable suspension for subcutaneous use. The approval covers its use as monotherapy or as adjunctive therapy alongside lithium or valproate for the maintenance treatment of bipolar I disorder in adults.
Previously approved for adult schizophrenia, UZEDY is administered once every one or two months. It is the first subcutaneous, long-acting risperidone formulation to utilize Medincell's proprietary SteadyTeq copolymer technology, which enables controlled and steady drug release. Therapeutic blood levels are achieved within 6 to 24 hours following a single dose. For bipolar I disorder, UZEDY is now available in three once-monthly dosing strengths: 50 mg, 75 mg, and 100 mg.
UZEDY was approved in the U.S. for the treatment of schizophrenia in adults in 2023.
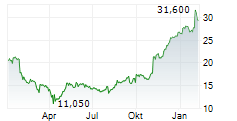
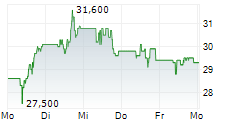
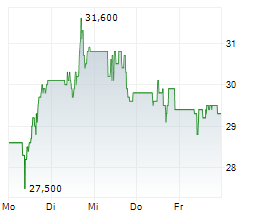
TEVA closed Friday's regular trading at $20.03 down $0.01 or 0.05%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News