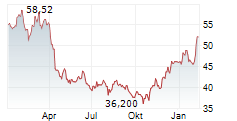
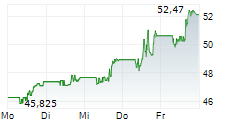
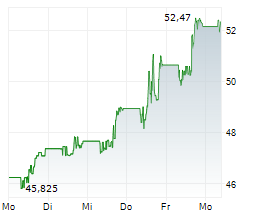
NEW YORK CITY (dpa-AFX) - SystImmune announced the treatment of the first patient in the IZABRIGHT-Breast01 study, a global Ph2/3 registrational study of izalontamab brengitecan, or iza-bren, in previously untreated triple negative breast cancer ineligible for anti-PD1 drugs. This milestone triggered a one-time payment of $250 million by Bristol Myers Squibb (BMY).
'Together with Bristol Myers Squibb, we have made significant progress in developing iza-bren globally. We remain deeply committed to delivering this potentially transformative therapy to patients with triple negative breast cancer and other solid tumors,' said Jie D'Elia, CEO, SystImmune.
Iza-bren is being developed by SystImmune's parent company, Sichuan Biokin Pharmaceutical Co. in China and jointly developed by SystImmune and Bristol Myers Squibb in territories outside of China.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News