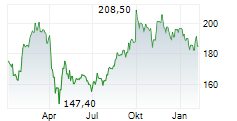
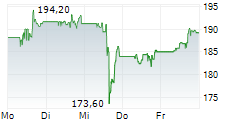
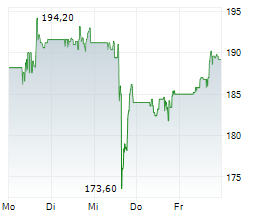
WASHINGTON (dpa-AFX) - AbbVie (ABBV) Monday announced the U.S. Food and Drug Administration (FDA) approval of a supplemental new drug application (sNDA) that updates the indication statement for RINVOQ (upadacitinib) for the treatment of adults with moderately to severely active ulcerative colitis (UC) and moderately to severely active Crohn's disease (CD).
Previously, RINVOQ was indicated for adults with moderately to severely active UC or CD who had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers. The updated indication statement also allows the use of RINVOQ for patients after they have received at least one approved systemic therapy in the event TNF blockers are clinically inadvisable.
'At AbbVie, we are committed to addressing the ongoing needs of patients living with inflammatory bowel disease,' said Kori Wallace, M.D., Ph.D., vice president, global head of immunology clinical development, AbbVie. 'Ulcerative colitis and Crohn's disease can impact every aspect of a patient's life. This label update gives healthcare providers the option to prescribe RINVOQ for patients with moderately to severely active inflammatory bowel disease after the use of one approved systemic therapy if TNF blockers are deemed clinically inadvisable by the prescribing physician.'
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News