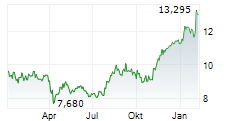
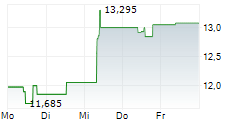
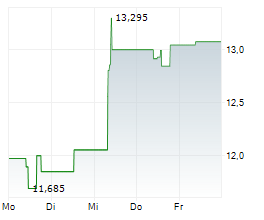
TOKYO (dpa-AFX) - Astellas Pharma Inc. (ALPMY) on Tuesday said the Phase 2 GLEAM study of zolbetuximab in metastatic pancreatic cancer failed to meet its primary endpoint of overall survival (OS).
The study was designed to evaluate the safety and efficacy of zolbetuximab in combination with gemcitabine plus nab-paclitaxel, compared with gemcitabine plus nab-paclitaxel alone, in patients with metastatic pancreatic adenocarcinoma.
The company noted that the impact of this result on Astellas' financial results for the fiscal year ending March 31, 2026, is expected to be minor.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News