PETAH TIKVA (dpa-AFX) - Formycon AG (FYB.F), Bioeq AG and Teva Pharmaceutical Industries Ltd. (TEVA) on Tuesday announced the launch of FYB201/Ranivisio or ranibizumab in Europe as the first Lucentis biosimilar available in pre-filled syringe presentation or PFS approved by European Medicines Agency for ophthalmic treatments.
FYB201/Ranivisio has been approved to treat severe visual impairments such as wet age-related macular degeneration or nAMD and other retinopathies.
Bioeq AG, a Swiss joint venture between Formycon and Polpharma Biologics Group BV, owns FYB201.
As per the deal, Bioeq's partner Teva will commercialize FYB201/Ranivisio pre-filled syringe across Europe.
The companies started the market launch of FYB201/Ranivisio PFS in France in October 2025, while additional countries, including Germany, will follow.
The Biosimilar product is currently available in a total of 21 countries in Europe, North America and the MENA region.
The pre-filled syringe presentation technology has been specifically designed for intravitreal injections, which refers to the injection of a medication into the vitreous body of the eye characterised for dosing accuracy, low injection pressure, and a minimized risk of application errors which are the key factors in the ophthalmic care.
The ready-to-use syringe reduces preparation time and supports efficient administration for patients with neovascular age-related macular degeneration and other serious retinal diseases.
Stefan Glombitza, CEO of Formycon, said, 'By introducing the first ranibizumab biosimilar in this modern pre-filled syringe presentation, we are setting new standards in convenience, safety and efficiency for ophthalmic treatments and are underlining our strong commitment to advancing ophthalmic care.'
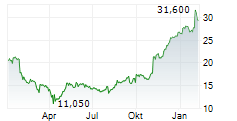
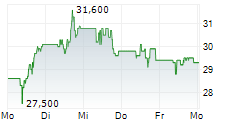
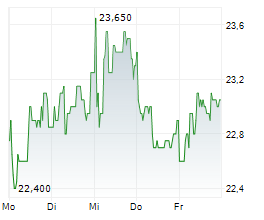
In the franfurt market, Formycon shares were trading 1.06% higher at 23.75 euros.
In the afterhours trading, TEVA shares were trading 0.10% lower at $19.40 on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News