TOKYO (dpa-AFX) - Astellas Pharma Inc. (ALPMY.PK), Wednesday announced new real-world preliminary data from the OPTION-VMS Phase IV study, a longitudinal, observational study in participants with VMS, also known as hot flashes and/or night sweats.
The findings demonstrated statistically significant improvements in VMS bother related to menopause, statistically significant improvements in subjective and objective sleep outcomes and significant improvements in activity impairment including overall work productivity.
Additionally, a preliminary analysis of work productivity outcomes from the study demonstrated that treating VMS associated with menopause with fezolinetant led to statistically significant improvements in Work Productivity and Activity Impairment questionnaire specific to VMS domains.
Shayna Mancuso D.O., FACOG, Head, US Medical Affairs, Women's Health-Fezolinetant, Astellas, commented, 'These new preliminary findings reinforce Astellas' commitment to advancing our understanding of fezolinetant beyond the pivotal clinical trials, with the goal of driving meaningful change for those affected by VMS.'
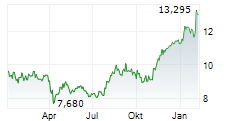
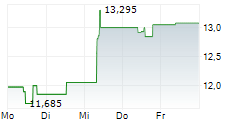
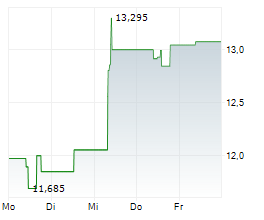
On Monday, ALPMY closed at $10.65, down 1.21 percent on the OTC Markets.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News