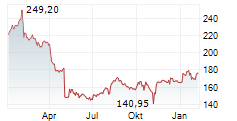
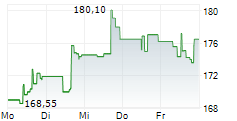
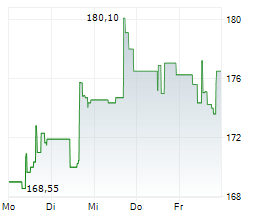
WASHINGTON (dpa-AFX) - Becton, Dickinson and Company (BDX), a medical technology firm, on Monday said it has received U.S. Food and Drug Administration (FDA) 510(k) clearance and Conformité Européenne (CE) marking in the European Union for its Enteric Bacterial Panel (EBP) and Enteric Bacterial Panel plus (EBP plus) for use on the BD COR System.
The BD COR System automates molecular lab workflows, supporting up to 1,650 tests and delivering up to 1,000 sample results in 24 hours with barcode-driven traceability and remote system management.
The BD EBP panels detect a broad range of enteric bacterial pathogens, including Salmonella spp., Campylobacter spp., Shigella spp./Enteroinvasive Escherichia coli (EIEC), and Shiga toxin-producing Escherichia coli (STEC). The panels use advanced Polymerase Chain Reaction (PCR) technology to streamline testing for a broad range of gastrointestinal (GI) bacterial pathogens from a single stool swab sample.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News