WASHINGTON (dpa-AFX) - Exact Sciences Corp. (EXAS), Friday announced the landmark clinical results from its ALTUS study, showing that the Oncoguard Liver blood test significantly outperforms ultrasound in detecting early-stage hepatocellular carcinoma or HCC, the most common type of liver cancer.
The results will be presented at the AASLD Liver Meeting on November 11, 2025, and later submitted for publication.
The 3,000-patient ALTUS trial, the largest of its kind in the U.S., found that Oncoguard Liver detected three times more early-stage cancers than ultrasound (67 percent vs 22 percent) and demonstrated superior sensitivity at very early stages (64 percent vs 9 percent). Overall test specificity reached 82 percent, exceeding clinical utility benchmarks.
Investigators said the findings mark a breakthrough for liver cancer surveillance, addressing long-standing challenges of ultrasound, such as inconsistent image quality and low screening adherence among at-risk patients. Dr. Binu John of the University of Miami called the test 'a game changer' for accessibility and equity in screening.
Exact Sciences Chief Medical Officer Dr. Paul Limburg said the data reaffirm the company's success in early cancer detection, following the impact of its Cologuard test for colorectal cancer. The Oncoguard Liver assay, developed with Mayo Clinic, uses a multiomic approach combining DNA methylation and protein markers to identify molecular signals of liver cancer.
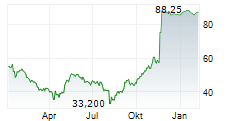
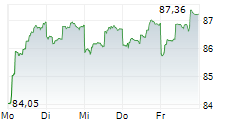
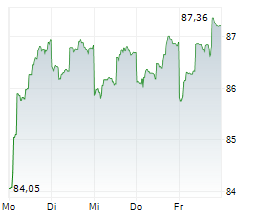
EXAS closed at $67.22, down 3.46 percent, and traded pre-market at $65.95, down 1.89 percent on the NasdaqCM.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News