Lund, Sweden - Clinical Laserthermia Systems AB (publ) ("CLS" or the "Company") today publishes its interim report for January - September 2025.
Highlights of the Third Quarter Interim Report
During the third quarter, CLS continued to strengthen its operational and financial position while producing and delivering products, development, and support services for growing the adoption and installed base of its product portfolio.
- The company achieved a key regulatory milestone in the US-FDA clearance of the expanded labeling of CLS ClearPoint Prism® branded Neuro Laser Therapy System to include both 3.0T and 1.5T MR image guidance and that significantly expands the market opportunity in neurosurgery.
- By strict cost control and higher operational efficiency, CLS delivered an improvement in operating profit of SEK 2,3 million compared to third quarter 2024.
- For the first nine months, CLS neuro business grew by 38 percent while total revenue grew by 18 percent.
- Revenue for the quarter amounted to SEK 2.8 million, a reduction compared with the same period last year, impacted mainly by timing of deliveries, late arrival of the expanded clearance for CLS ClearPoint Prism® branded Neuro Laser Therapy System at the end of the quarter, and lower sales in the urology segment.
- Following the strategic decision to close CLS direct sales operation within the urology segment by end of 2024, the revenue declined by 61% compared to the third quarter of 2024.
- Operating profit improved by SEK 21.4 million for the first nine months compared with the same period last year.
- CLS strengthened its financial base through the full subscription of warrants of series TO8B, adding SEK 20.9 million in gross proceeds. Combined with the capital raised in February, this completes the SEK 37 million financing package announced earlier this year, aimed at ensuring that CLS is capitalized to execute its growth strategy.
- The strengthening of the Swedish Krona during 2025 has impacted the intercompany loans, YTD revaluation is SEK 11,4 million. The Q3 reporting also includes a correction of SEK 789 thousand for late received supplier invoices from 2024.
- CLS remains debt-free and maintains a positive operational cash flow trend supported by lower operating expenses.
Summary of the interim report (relates to the Group)
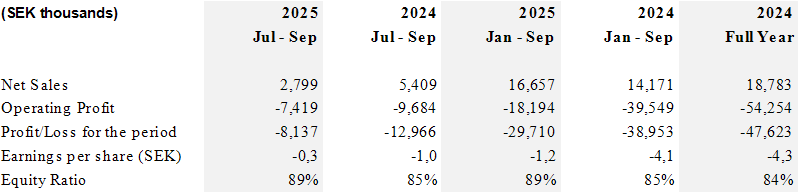
Comments from CEO Dan J. Mogren
In September, we achieved a key milestone when the U.S. Food and Drug Administration (FDA) granted expanded clearance for CLS ClearPoint Prism® branded Neuro Laser Therapy System to include both 3.0T and 1.5T MRI scanners. This approval more than doubles the addressable U.S. market for Neuro LITT procedures and positions CLS and ClearPoint Neuro for accelerated adoption in hospitals that previously could not access the technology.
The clearance represents not only an important commercial inflection point but also validation of the clinical versatility of our laser therapy platform. The first installations of the 1.5T labeled PRISM system are expected in the fourth quarter of this year.
In early October we announced positive results from the clinical safety study on laser ablation, performed using CLS Prism branded LITT platform in patients with malignant brain tumor. The study was conducted at Skåne University Hospital and presented in October at the CNS annual meeting in Los Angeles.
Patients in the study showed increased median survival compared to a matched control group treated with open surgery. We are very pleased that the primary clinical safety objectives of the study were successfully achieved and by positive feedback from the neurosurgeons that the system is easy to use with a reproducible workflow enabling a median ablation time of 6.5 minutes.
Further empowered by the recent successful regulatory and clinical study achievements, and in awareness of the current challenging trade climate, we have intensified conversations with our upstream and downstream partners to align on an accelerated growth scenario going forward.
In line with our growth strategy, the company has decided to actively start expanding the partnership model outside of neurosurgery. This decision aims to broaden the company's commercial base and create a diversified, more balanced and predictable growth platform going forward.
Looking ahead, our priorities remain clear:
- Support a broader U.S. adoption of the ClearPoint Prism® system under the expanded FDA clearance including both 3.0T & 1.5T MRI image guidance and through the commercial program of our partner ClearPoint Neuro.
- Advance prioritized Prism improvements, and regulatory initiatives in Europe, to further expand and strengthen our market position in neurosurgery.
- Expand our partnership model outside of neurosurgery to capture additional business opportunities, reduce risk and add stability in our business.
- Continue optimizing margins and cost structure in our move toward sustainable profitability.
With strong clinical momentum, lowered barriers to adoption and market expansion, a focused organization, and a solid financial foundation, CLS is well positioned to deliver on its mission - to make minimally invasive laser therapy available to more patients worldwide.
Thank you for your continued support, and we look forward to your active participation in the exciting developments to come.
Dan J. Mogren
CEO, Clinical Laserthermia Systems AB
Significant events in the third quarter of 2025
- CLS announced that the U.S. Food and Drug Administration (FDA) has granted clearance for expansion of the labeling of ClearPoint Prism® Neuro Laser Therapy System. In addition to the existing clearance for use with 3.0 Tesla (3.0T) magnetic resonance imaging (MRI) guidance, the system is now also cleared for use with 1.5T MRI guidance. The expanded clearance significantly broadens the accessibility of the Prism Neuro Laser Therapy System for U.S. hospitals and patients, as approximately half of all MRI-guided neurosurgical procedures are performed using 1.5T scanners. The approval thereby more than doubles the addressable U.S. market for Neuro Laser Interstitial Thermal Therapy (Neuro LITT) procedures.
Significant events after the end of the period
- CLS raised SEK 20.9 million through full subscription of Warrants of Series TO 8B. All 5,500,000 warrants of series TO 8B, issued in connection with the directed share issue in February 2025, have been exercised. The exercise added SEK 20.9 million before transaction costs to CLS, corresponding to a subscription rate of 100 percent. The total number of shares in CLS will amount to 31,166,594, corresponding to a share capital of approximately SEK 71,981,747.85.
- CLS announced positive results from a clinical safety study on laser ablation, performed using CLS proprietary LITT platform in patients with malignant brain tumor, conducted at Skåne University Hospital. Patients had increased median survival compared to a matched control group treated with open surgery. The study's primary objective - to investigate whether the CLS proprietary LITT platform was safe and feasible in the treatment of brain tumors - was successfully achieved. The study, that was conducted by neurosurgeons at Skåne University Hospital, was sponsored by CLS and co-funded by ClearPoint Neuro, Inc. The laser ablations were guided by 3T MR imaging and neuro navigation was performed using ClearPoint® Neuro Navigation System, ClearPoint Neuro Inc.
For more information, please contact:
Dan J. Mogren, CEO Clinical Laserthermia Systems AB (publ)
Phone: +46 (0)705 90 11 40
E-mail: dan.mogren@clinicallaser.com
About CLS
Clinical Laserthermia Systems AB (publ), develops and sells TRANBERG® Thermal Therapy System and ClearPoint Prism® Neuro Laser Therapy System with sterile disposables, for minimally invasive treatment of cancer tumors and drug-resistant epilepsy. The products are marketed and sold through partners for image-guided laser ablation. CLS is headquartered in Lund, Sweden, with subsidiaries in Germany, the United States and a marketing company in Singapore. CLS is listed on Nasdaq First North Growth Market under the symbol CLS B. Certified adviser (CA) is FNCA Sweden AB.
For more information about CLS, please visit the Company's website: www.clinicallaser.se
This disclosure contains information that CLS is obliged to make public pursuant to the EU Market Abuse Regulation (EU nr 596/2014). The information was submitted for publication, through the agency of the contact person, on 14-11-2025 08:30 CET.