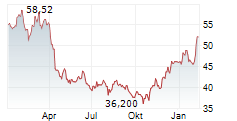
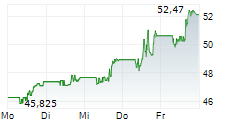
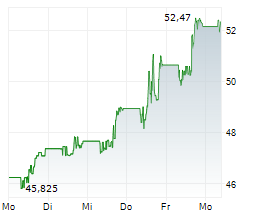
NEW BRUNSWICK (dpa-AFX) - Bristol-Myers Squibb Co. (BMY) on Friday said it has halted the Phase 3 Librexia ACS trial of milvexian, conducted in partnership with Johnson & Johnson (JNJ), in patients who recently experienced an acute coronary syndrome (ACS) event.
The decision follows an interim analysis conducted by the Independent Data Monitoring Committee, which found the study was unlikely to meet its primary efficacy endpoint.
The Librexia clinical trial program includes two additional Phase 3 studies-Librexia AF for atrial fibrillation and Librexia STROKE for secondary stroke prevention. The monitoring committee recommended these trials proceed as planned, with topline results expected in 2026.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News