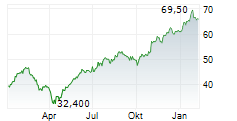
WESTON (dpa-AFX) - Sandoz announced that TYRUKO or natalizumab-sztn is available to patients in the US. Developed by Polpharma Biologics, TYRUKO is the only FDA approved natalizumab biosimilar for the treatment of relapsing forms of multiple sclerosis. TYRUKO is approved by the FDA as monotherapy to treat all indications covered by reference medicine Tysabri.
Sandoz entered into a global commercialization agreement for biosimilar natalizumab in 2019. Under the agreement, Polpharma Biologics will maintain responsibility for the development of the medicine, manufacturing and supply of the drug substance. Sandoz has the rights to commercialize and distribute it in all markets. TYRUKO is now available in 14 European countries.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News