WASHINGTON (dpa-AFX) - Becton, Dickinson and Company (BDX), Monday the Conformite Europeenne or CE Marked BD Onclarity HPV Assay for the BD COR System and the BD Viper LT System have been accepted for the World Health Organization list of prequalified in vitro diagnostic products.
This WHO pre-qualification marks an important step towards changing the future of cervical cancer screening, especially in low- and middle-income countries.
The BD Onclarity HPV Assay is approved for self-collection, including at-home self-collection in countries that recognize the CE mark, enabling improved access, especially in settings with limited resources.
The BD Viper LT System supports flexible testing in decentralized settings, whereas the BD COR System offers high-throughput automation for centralized laboratories.
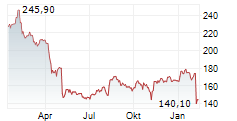
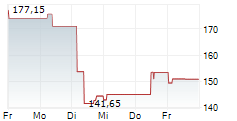
In the pre-market hours, BDX is trading at $193.07 without any movement on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News