WASHINGTON (dpa-AFX) - Genmab A/S (GMAB) announced that the U.S. Food and Drug Administration approved EPKINLY (epcoritamab-bysp) in combination with rituximab and lenalidomide (EPKINLY + R2) for the treatment of adult patients with relapsed or refractory (R/R) follicular lymphoma (FL).
This approval marks the third indication for EPKINLY and the first-ever FDA approval for a bispecific combination therapy in the lymphoma space.
In June 2024, EPKINLY monotherapy was granted accelerated approval by the FDA for the treatment of R/R FL following two or more lines of systemic therapy. With the results from the confirmatory Phase 3 EPCORE FL-1 study, the FDA has also converted the accelerated approval into a full approval.
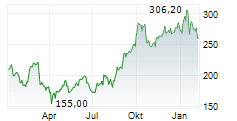
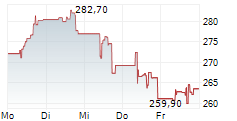
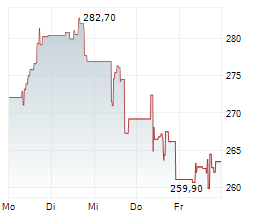
GMAB closed Tuesday's regular trading at $30.32, down $0.10 or 0.33% on NasdaqGS. But in after-hours trading, the stock edged up by $0.04, or 0.13%.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News