WASHINGTON (dpa-AFX) - DexCom, Inc. (DXCM) on Wednesday announced that the U.S. Food and Drug Administration (FDA) has cleared Dexcom Smart Basal, the first and only continuous glucose monitor (CGM)-integrated basal insulin dosing optimizer designed for adults with Type 2 diabetes using glargine U-100 long-acting insulin therapy.
The system uses data from the Dexcom G7 sensor and logged insulin doses to provide daily personalized recommendations for adjusting long-acting insulin, under the guidance of a healthcare provider. Dexcom Smart Basal will be available as part of the Dexcom G7 15-Day experience shortly after its U.S. launch.
'Dexcom Smart Basal is designed to remove barriers to insulin initiation-giving healthcare providers the confidence to start their patients on basal therapy sooner and with greater ease,' said Peter Simpson, senior vice president of innovation and sensor technology at Dexcom.
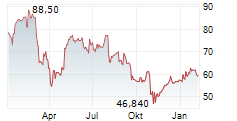
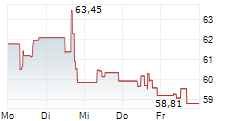
DexCom shares were down 0.28% in pre-market trading after closing at $60.17, up 2.87% on Tuesday.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News