NEW YORK CITY (dpa-AFX) - Bristol Myers Squibb (BMY), on Tuesday, announced that it has received approval from the European Commission to expand the use of its CAR T cell therapy Breyanzi for relapsed or refractory mantle cell lymphoma. The approval applies to all European Union member states as well as the European Economic Area countries Iceland, Norway and Liechtenstein.
Mantle cell lymphoma, or MCL, is an aggressive, rare form of non-Hodgkin lymphoma that originates in the mantle zone of the lymph node, frequently affecting elderly males more than females, with an average age at diagnosis in the mid-60s.
The approval is based on results from the MCL cohort of TRANSCEND NHL 001, which enrolled adult patients with relapsed or refractory mantle cell lymphoma who had received a minimum of 2 prior lines of treatment, including Bruton's Tyrosine Kinase (BTK) inhibitors.
TRANSCEND NHL 001 is a phase I single-arm, design study to determine the safety, pharmacokinetics and antitumor activity of Breyanzi in adult patients with relapsed or refractory B-cell non-Hodgkin lymphoma, including diffuse large B-cell lymphoma, high-grade B-cell lymphoma, primary mediastinal B-cell lymphoma, follicular lymphoma Grade 3B and mantle cell lymphoma.
According to the results from the MCL cohort, among patients treated in the third-line plus setting with Breyanzi, the overall response rate was 82.7% and the complete response rate was 71.6%. After 24 months, about 50.8% of respondents were still responding, indicating that the effect can persist.
Breyanzi is already approved in Europe for treating relapsed or refractory diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma (HGBCL), primary mediastinal large B-cell lymphoma (PMBCL), and follicular lymphoma.
In the U.S, the drug is approved for the treatment of adult patients with relapsed or refractory large B-cell lymphoma, chronic lymphocytic leukemia or small lymphocytic lymphoma, follicular lymphoma, and mantle cell lymphoma.
Breyanzi generated global sales of $966 million in the nine months ended Sep.30, 2025, compared to $484 million in the year-ago period.
Commenting on the approval, Emma Charles, senior vice president, Europe Region, Bristol Myers Squibb, said, 'While frontline therapies have advanced over the years for this rare but aggressive form of non-Hodgkin lymphoma, the vast majority of patients relapse or become resistant, and face reduced survival outlook, leaving a critical need for new treatment options. Breyanzi has the opportunity to address a treatment gap for this patient population based on its demonstrated clinical benefit.'
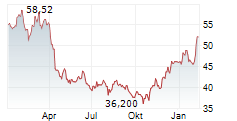
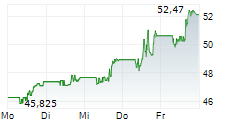
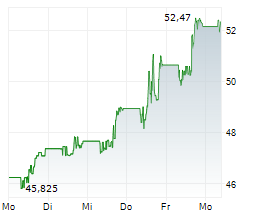
BMY has traded in a range of $42.52 to $63.33 in the last 1 year. The stock closed yesterday's trading at $47.76, up 3.26%.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News