TOKYO (dpa-AFX) - Japan-based Otsuka Pharmaceutical Co., Ltd., a wholly owned subsidiary of Otsuka Holdings Co., Ltd. (4578.T, OTSKF), has submitted a New Drug Application to the FDA for Centanafadine, its drug candidate for the treatment of attention-deficit hyperactivity disorder in children, adolescents, and adults.
Centanafadine, designed as a once daily extended-release capsule, is a norepinephrine, dopamine, and serotonin reuptake inhibitor to treat ADHD.
Attention-deficit hyperactivity disorder (ADHD) is a chronic neurodevelopmental disorder characterised primarily by impairments in attention, hyperactivity, and impulsivity, with an estimated 15.5 million adults in the U.S. currently diagnosed with ADHD. As per the CDC report, about 7 million U.S. children aged 3-17 years, approximately 11.4 % have been diagnosed with ADHD.
According to a report by Grand View Research, the global attention deficit hyperactivity disorder market size was estimated at $14.3 billion in 2023 and is expected to reach $18.6 billion by 2030, growing at a CAGR of 3.7% from 2024 to 2030.
Otsuka submitted the New Drug Application based on results from a phase 3 clinical program that consisted of four different pivotal phase 3 trials that evaluated the efficacy and safety of Centanafadine across children, adolescents, and adults.
The FDA has a 60-day window after NDA submission to decide if it will accept the application for review.
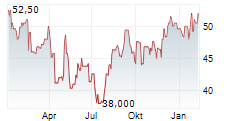
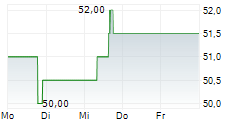
OTSKF has traded in a range of $44.40 to $57.99 over the past year on the OTC market. The stock closed yesterday's trading at $57.75. On the Tokyo market, the shares were trading 1.09% higher at 8660 yen.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News