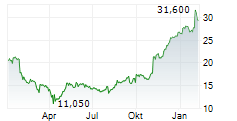
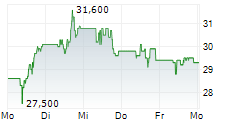
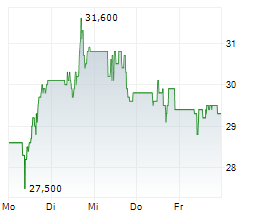
PETAH TIKVA (dpa-AFX) - Teva Pharmaceuticals International, a subsidiary of Teva Pharmaceutical Industries (TEVA), announced that the European Commission has granted marketing authorizations for its two denosumab biosimilar candidates PONLIMSI, a biosimilar to Prolia and DEGEVMA, a biosimilar to Xgeva. Teva plans to launch both products in key European markets in the coming months.
Steffen Nock, SVP Head of Biosimilars & Chief Science Officer, said: 'This approval represents an important step forward in increasing patient access to biosimilar therapies for serious bone conditions.'
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News