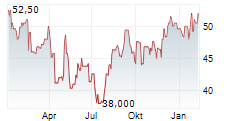
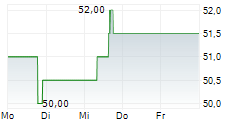
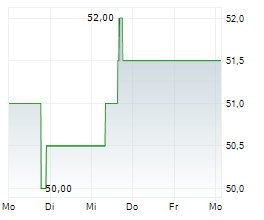
CANBERA (dpa-AFX) - Otsuka Pharmaceutical Co., Ltd. and Otsuka Pharmaceutical Development & Commercialization, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval of VOYXACT (sibeprenlimab-szsi). The therapy is indicated for reducing proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) who are at risk of disease progression.
VOYXACT is a self-administered subcutaneous injection, dosed once every four weeks. The approval was based on interim results from the VISIONARY Phase 3 clinical trial, where VOYXACT demonstrated a significant placebo-adjusted treatment effect-achieving a 51% reduction in proteinuria at nine months of treatment (50% with VOYXACT vs. 2% with placebo, n=320).
Importantly, VOYXACT is the first and only therapy designed to block A-Proliferation-Inducing-Ligand (APRIL), representing a novel mechanism of action in the treatment of IgAN.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News