Highlights July-September 2025
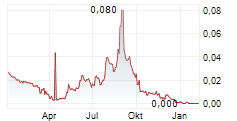
- Net sales and operating loss for Q3 2025 in line with expectations: Net sales of 101 KSEK for the period (235) largely reflected royalty revenue. Operating loss was 25.5 MSEK, down from 31.2 MSEK in the third quarter of 2024. Earnings per share before and after dilution were -0.10 SEK (-0.73).
- Cash burn lower than guidance: Cash flow from operating activities was -16.1 MSEK, reduced from -17,3 MSEK in Q3 2024. The cash burn rate of 5.4 MSEK per month was well below the Company's previously communicated guidance of 8 to 10 MSEK per month, as we reduced spending in July and August prior to the September launch of PancreaSure. Cash and cash equivalents at end of period equaled 26.6 MSEK (54.2), supported by a bridge loan of 19.0 MSEK.
- Successful commercial launch with strong early interest from key opinion leaders: PancreaSure was launched on September 2nd, following rigorous validation, and embraced by leading pancreatic cancer experts at seven top U.S. surveillance programs, including University of Pennsylvania, Northwestern University, and Hackensack Meridian, who began ordering tests for their high-risk patients in line with Immunovia's KOL-focused commercial strategy.
- Continued validation of PancreaSure within the scientific community: The analytical validation study received a "Distinguished Abstract Award" from the Academy of Diagnostics & Laboratory Medicine, a distinction reserved for only nineteen of the more than 800 abstracts submitted. This demonstrates the growing interest and confidence from the medical and academic communities in PancreaSure's role in early pancreatic cancer detection.
- Secured manufacturing independence and significant reduction in cost of goods sold: The licensing agreement reached with Proteomedix reduces supply risk and improves gross margin.
- Continued progress toward Medicare reimbursement: The Centers for Medicare & Medicaid Services (CMS) issued a preliminary payment determination for PancreaSure of $897. To accelerate progress toward full Medicare coverage, Immunovia strengthened its market access capabilities with the appointment of Natalie Carfora as VP of Market Access and Reimbursement.
- Excellent accuracy demonstrated in late-stage pancreatic cancer and healthy controls: In the AFFIRM clinical validation study, announced September 24, 2025, the PancreaSure test detected 87.9% of Stage III and IV pancreatic cancers. The test showed specificity of 97.7%, avoiding false positive results in blood samples from healthy controls.
Significant events after the period
- CAP accreditation confirms operational excellence: Immunovia's North Carolina laboratory received accreditation from the College of American Pathologists (CAP), confirming that operations meet the highest standards for quality, accuracy, and patient safety.
- Growing scientific validation through peer-reviewed publication and conference presentations: The CLARITI study was published in Gastroenterology, the leading journal for gastrointestinal disease. This prestigious publication provides independent peer-reviewed validation of the PancreaSure test's performance. During the fall conference season, Immunovia presented clinical validation data at the American College of Gastroenterology Annual Scientific Meeting and the Inherited Gastrointestinal Cancers Meeting, reinforcing medical community interest and building advocacy among key opinion leaders.
- Successful rights issue: On October 23, the Company announced the outcome of the rights issue, with 293,632,417 shares, corresponding to approximately 87.9 percent of the rights issue subscribed for, with and without exercise of subscription rights. Accordingly, 40,276,397 shares, corresponding to approximately 12.1 percent of the rights issue, were allotted to the guarantors, whereby the rights issue was subscribed to 100 percent in aggregate. Immunovia received proceeds of approximately SEK 100 million before deduction of costs attributable to the rights issue.
"This quarter we launched the PancreaSure test, a breakthrough product that brings us closer to achieving our mission of saving lives through early detection of pancreatic cancer."
Jeff Borcherding, CEO and President, Immunovia AB
Conference call
Immunovia will hold a webcast teleconference at 15:00 CET on November 26 with Jeff Borcherding, CEO and President.
To take part of the presentation, please dial one of the numbers or watch via the web link below.
Sweden: +46 (0)8 5051 0031
United Kingdom: +44 (0) 207 107 06 13
United States: +1 (1) 631 570 56 13
Link to the webcast:
https://creo-live.creomediamanager.com/a62cd97b-ff6a-4627-9cec-575b30bce20
For further information, please contact
Jeff Borcherding, CEO
jeff.borcherding@immunovia.com
Immunovia in brief
Immunovia AB is a diagnostic company whose mission is to increase survival rates for patients with pancreatic cancer through early detection. Immunovia is focused on the development and commercialization of simple blood-based testing to detect proteins and antibodies that indicate a high-risk individual has developed pancreatic cancer. Immunovia collaborates and engages with healthcare providers, leading experts and patient advocacy groups to make its test available to individuals at increased risk for pancreatic cancer.
USA is the world's largest market for detection of pancreatic cancer. The Company estimates that in the USA, 1.8 million individuals are at high-risk for pancreatic cancer and could benefit from annual surveillance testing.
Immunovia's shares (IMMNOV) are listed on Nasdaq Stockholm.
For more information, please visit www.immunovia.com.