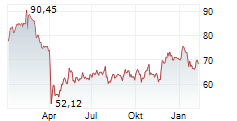
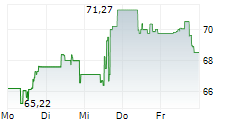
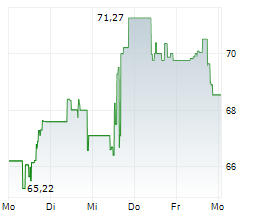
WASHINGTON (dpa-AFX) - GE HealthCare Technologies, Inc. (GEHC) announced Wednesday that it has received FDA Premarket Authorization for Pristina Recon DL, an advanced 3D mammography image reconstruction technology. Powered by deep learning, Pristina Recon DL sets a new standard in image definition and sharpness.
Breast cancer remains one of the most common cancers among women, with one in eight expected to face a diagnosis in their lifetime and an estimated 1.1 million breast cancer-related deaths projected annually by 2050.
As the burden of disease continues to rise, the advancement of AI-powered technologies holds remarkable promise in supporting early detection, accurate diagnosis and helping elevate the quality of care.
Pristina Recon DL leverages two deep learning models working in sequence to enable separation of meaningful signal from noise. The first model reconstructs high fidelity 3D volumes with greater purity, minimizing artifacts and perceived noise. The second model is trained to enhance the visualization of clinically relevant information in the DL synthesized 2D view.
Pristina Recon DL is an enhancement to GE HealthCare's Pristina Via system. It is the first mammography technology to use deep learning in combination with iterative reconstruction to provide outstanding digital breast tomosynthesis (DBT) image quality without compromising on patient dose.
Pristina Via with Recon DL3 utilizes NVIDIA RTX accelerated computing technology to execute its advanced image reconstruction, delivering fast and accurate images in the exam room and for clinical diagnosis.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News