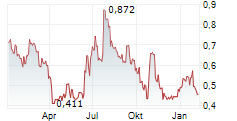
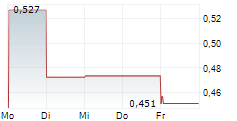
Mendus has reported encouraging long-term data from its Phase I ALISON trial, which is investigating its lead off-the-shelf cancer vaccine, vididencel, in high-risk ovarian cancer (OC). The latest update reflects outcomes following two years of follow-up, ultimately confirming the safety and tolerability of vididencel, as there were no product-related serious side effects. Furthermore, it was reported that eight of 17 patients were alive following the two-year follow-up, with improved survival associated with tumour-directed immune responses following treatment with vididencel. These outcomes highlight vididencel's potential as an active immunotherapy for this indication. Management also noted that the results serve as a basis to explore vididencel with novel combination approaches to overcome immune evasion mechanisms, although given Mendus's recently renewed clinical strategy, further development efforts will be subject to securing an appropriate partner.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research