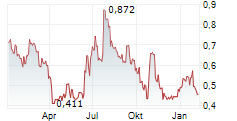
Mendus has presented incremental clinical data for vididencel at the American Society of Hematology (ASH) 67th Annual Meeting, involving long-term follow-up for the ADVANCE II trial. This study is testing the ability of the candidate to prolong survival in acute myeloid leukaemia (AML) as a maintenance therapy. The data showed that at a median follow-up of 55 months, 13/20 patients treated with vididencel were alive, with eight patients having passed the five-year follow-up, and overall survival at 63%. (The prior update was at a median follow-up of 48 months, where 13/20 patients were alive, with five having reached the five-year follow-up). Immunological data tracked with survival, consistent with prior readouts, adding further confidence in vididencel as an active immunotherapy against residual disease in AML. Importantly, we believe this update supports Mendus's renewed clinical strategy for the candidate, which is set to position it more broadly in this disease area.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research