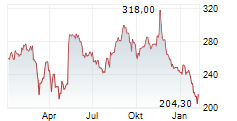
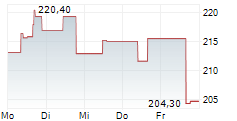
BRUSSELS/FRANKFURT/PARIS (dpa-AFX) - Insulet (PODD) announced it has received FDA 510(k) clearance for significant enhancements to the Omnipod 5 Automated Insulin Delivery System. The new 100 mg/dL Target Glucose expands Omnipod 5's customization range to six settings between 100-150 mg/dL in 10 mg/dL increments. The updates to the Omnipod 5 algorithm are projected to launch in the United States in the first half of 2026, where Omnipod 5 is cleared for people aged 2 and older with type 1 and aged 18 and older with type 2 diabetes.
'This is the most significant algorithm advancement to our Omnipod 5 System since its launch in 2022,' said Eric Benjamin, Insulet COO.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News