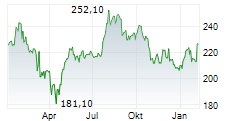
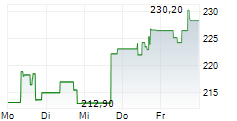
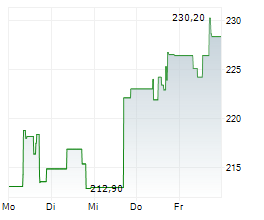
WASHINGTON (dpa-AFX) - Resmed (RMD, RMD.AX), Monday announced it has received U.S. Food and Drug Administration (FDA) clearance for Personalized Therapy Comfort Settings (PTCS), to be marketed as Smart Comfort.
Smart Comfort is the first FDA-cleared AI-enabled medical device that recommends personalized comfort settings to help people with obstructive sleep apnea (OSA) start and stay on CPAP therapy.
Smart Comfort will launch in early 2026 in a limited U.S. beta version for new users of myAir, Resmed's consumer sleep companion app, paired with a Resmed AirSense 11 device. It will be followed by a broader U.S. rollout to new myAir users later in 2026.
CPAP therapy is not one-size-fits-all. Addressing common therapy issues, like comfort and mask fit, early can promote long-term adherence. Smart Comfort leverages Resmed's proprietary machine-learning algorithms, drawing on more than 100 million nights of de-identified, real-world sleep data and user information, such as age, gender and Apnea-Hypopnea Index (AHI), to recommend individualized comfort settings for CPAP therapy delivered by Resmed's market leading AirSense 11 devices.
'For people new to CPAP therapy, personalized comfort settings can help them adjust faster and more comfortably, improving confidence and overall health,' said Justin Leong, Chief Product Officer at Resmed. 'Smart Comfort's FDA clearance marks an important milestone for the future of personalized, data-driven care. It's another example of how we're using technology and real-world evidence to make sleep health more personal, accessible and effective.'
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News