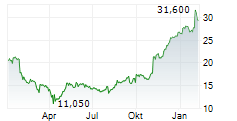
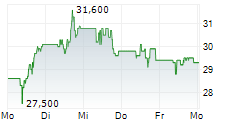
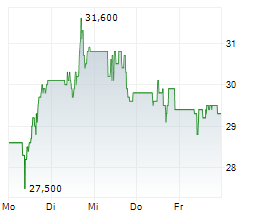
PETAH TIKVA (dpa-AFX) - Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (TEVA), announced the submission of a New Drug Application to the FDA for olanzapine extended-release injectable suspension for the treatment of schizophrenia in adults. The NDA for olanzapine Olanzapine long-acting injectable is based on results from the Phase 3 SOLARIS trial. Olanzapine long-acting injectable utilizes SteadyTeq, a copolymer technology proprietary to Medincell that provides a controlled steady, sustained release of olanzapine.
'The innovation of olanzapine LAI comes from its delivery of olanzapine, a foundational treatment for schizophrenia, as a once-monthly subcutaneous formulation, said Eric Hughes, Executive Vice President, Global R&D and Chief Medical Officer at Teva.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News