NEW YORK CITY (dpa-AFX) - BioNTech SE (BNTX) and Bristol-Myers Squibb Company (BMY), Tuesday announced the first interim data from a global randomized Phase 2 trial, evaluating pumitamig in patients with locally advanced/metastatic triple-negative breast cancer.
The trial evaluated pumitamig in two dose levels and in combination with four different chemotherapeutic agents in the first- and second-line treatment of participants.
In Cohort 1, patients received pumitamig plus nab-paclitaxel, whereas patients in Cohort 2 received the flat-dose equivalent of 20 mg/kg in combination with three different chemotherapy regimens.
Among 39 efficacy-evaluable first-line and second-line patients in Cohort 1, the confirmed objective response rate was 61.5 percent, the unconfirmed objective response rate was 71.8 percent, and the disease control rate was 92.3 percent.
Moreover, the progression-free survival rate at 9 months was 59.3 percent.
Anne Kerber, Senior Vice President, Head of Development, Hematology, Oncology, Cell Therapy at Bristol Myers Squibb, commented, 'The encouraging results are especially meaningful in patients with PD-L1 low or negative tumors (CPS<10), representing the potential of pumitamig to deliver meaningful benefit across PD-L1 expression levels, including patients who historically have had fewer effective treatments.'
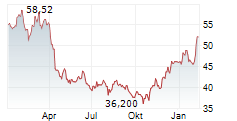
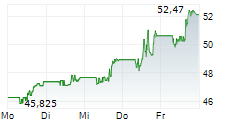
In the pre-market hours, BNTX is trading at $97.30, up 0.02 percent on the Nasdaq, and BMY is trading at $51.89, up 0.38 percent on the New York Stock Exchange.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News