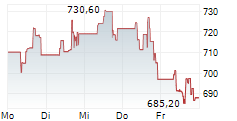
WASHINGTON (dpa-AFX) - Argenx SE (ARGX ARGX.BR) announced on Monday that it is discontinuing its Phase 3 UplighTED studies evaluating Efgartigimod PH20 SC in adults with moderate to severe thyroid eye disease, based on the Independent Data Monitoring Committee's recommendation. Shares are down over 6% in premarket trading.
The Independent Data Monitoring Committee (IDMC), after conducting a futility evaluation of unblinded data from patients completing 24 weeks in the Phase 3 UplighTED studies and reviewing data from a pre-specified interim analysis, recommended stopping the trials for futility.
Thyroid Eye Disease (TED) is an autoimmune condition, often linked to Graves' disease, affecting about 19 per 100,000 people in the U.S., predominantly women, causing symptoms like bulging eyes, dryness, pain, and double vision. Unlike the standard treatments for Thyroid Eye Disease, which often include steroids or surgery, Efgartigimod PH20 SC was expected to target the immune system more precisely.
Efgartigimod PH20 SC, marketed under the brand name VYVGART Hytrulo, is already approved by the FDA for the treatment of myasthenia gravis and chronic inflammatory demyelinating polyradiculoneuropathy.
Luc Truyen, M.D., PhD, Chief Medical Officer at argenx, said they had well planned this futility analysis, as it provides a meaningful interim evaluation of observed patient outcomes and enables them to assess the study's future likelihood of success responsibly.
After closing and locking the database, Argenx will conduct a comprehensive analysis of the data to understand outcomes better and guide future TED research. Findings will be shared at an upcoming medical meeting.
The company also markets VYVGART, which is approved by the FDA for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive.
VYVGART contains the active ingredient efgartigimod alfa and is given via intravenous infusion, while VYVGART Hytrulo is a coformulation of efgartigimod alfa and hyaluronidase and is administered as a more convenient subcutaneous injection.
The product net sales of VYVGART in the third quarter of 2025 were $1.13 billion compared to $0.6 billion in the year-ago period.
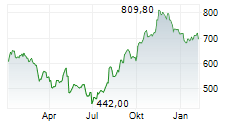
ARGX closed Friday's trade 1.30% lower at $877.94.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News