WASHINGTON (dpa-AFX) - Elanco Animal Health Incorporated (ELAN) on Thursday said it has received conditional approval from the U.S. Food and Drug Administration (FDA) for Credelio Quattro CA1 for the treatment of infestations caused by New World screwworm larvae in dogs.
The conditional approval was based on a study evaluating the efficacy of lotilaner, the active ingredient in Credelio Quattro CA1, against New World screwworm. Published in Parasites & Vectors, the study found that oral administration of lotilaner at the minimum recommended dosage was 100% effective against Cochliomyia hominivorax larvae within 24 hours in naturally infested dogs.
According to the U.S. Department of Agriculture, 14 cases of New World screwworm have been reported within 400 miles of the U.S.-Mexico border, all linked to cattle movement.
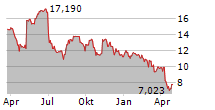
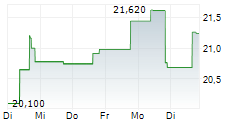
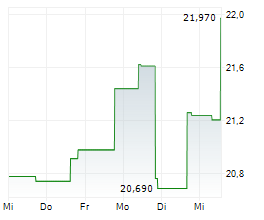
ELAN shares were up more than 2% in pre-market after closing at $22,08, down 1.82%.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News