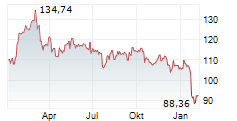
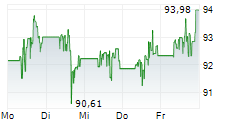
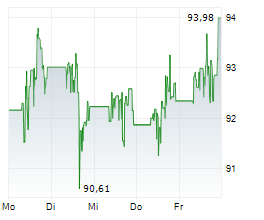
NORTH CHICAGO (dpa-AFX) - Abbott (ABT) Thursday said it has received U.S. Food and Drug Administration clearance and CE Mark approval for its Amplatzer Piccolo Delivery System, designed to treat premature babies with a common heart defect known as patent ductus arteriosus (PDA).
The system is used with Abbott's Amplatzer Piccolo Occluder and is designed for extremely small patients, including infants weighing as little as two pounds. PDA occurs when a blood vessel that should close after birth remains open, sending excess blood to the lungs and making breathing difficult.
According to Abbott, the new delivery system simplifies the procedure by requiring only one catheter instead of multiple tools. Its shorter, softer design allows for more precise placement in fragile patients, helping reduce procedural risks.
'This is a major step forward in treating PDA in premature infants,' said Dr. Evan Zahn, professor of cardiology and pediatrics at Cedars-Sinai Medical Center. 'The system improves precision and confidence when treating very small and vulnerable patients.'
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News