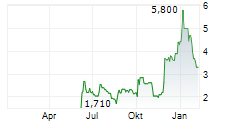
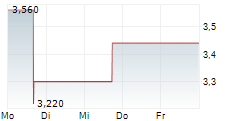
VANCOUVER, British Columbia, Jan. 08, 2026 (GLOBE NEWSWIRE) -- NervGen Pharma Corp. ("NervGen" or the "Company") (TSXV: NGEN) (NASDAQ: NGEN), a clinical-stage biopharmaceutical company developing first-in-class neuroreparative therapeutics for spinal cord injury (SCI) and other neurotraumatic and neurologic conditions, today announced the Company's common shares have been approved for listing on Nasdaq. NervGen's common shares will begin trading on Nasdaq today, under the symbol "NGEN."
"A listing on Nasdaq marks a defining moment in NervGen's evolution as a leading biopharmaceutical company developing neuroreparative therapeutics," said Adam Rogers, MD, Interim Chief Executive Officer of NervGen Pharma. "With unprecedented Phase 1b/2a CONNECT SCI data demonstrating durable improvements in function, independence, and quality of life in chronic SCI, we are entering late-stage development from a position of clinical and organizational strength. We believe this listing will enhance visibility in the marketplace, improve liquidity, broaden and diversify our shareholder base, and ultimately drive long-term shareholder value as we advance NVG-291 toward potentially becoming the first approved pharmacologic therapy for SCI and transforming the treatment paradigm for neurotraumatic and neurologic conditions with significant unmet medical need."
NervGen's listing on Nasdaq follows the Company's announcement of expanded Phase 1b/2a CONNECT SCI Study dataon November 24th, 2025, in which NVG-291 demonstrated unprecedented durable improvement in function, independence, and quality of life in individuals with chronic SCI between 1 to 10 years post-injury. NVG-291's ability to enable the nervous system to repair itself is supported by statistically significant improvements in upper-limb corticospinal signaling, as well as statistically significant reductions in hyperactive upper and lower-limb reticulospinal signaling. NervGen will conduct a U.S. Food and Drug Administration (FDA) End-of-Phase 2 meeting in early 2026 to align on the development and registration pathway for NVG-291. In September 2025, the Company completed an FDA Type C meeting, during which the FDA confirmed that multiple regulatory pathways are available to support approval.
About NervGen Pharma
NervGen Pharma Corp.in individuals with chronic SCI between 1 to 10 years post-injury and is the first pharmacologic candidate to demonstrate durable improvement in function, independence, and quality of life. Enrollment of individuals with subacute SCI in the Phase 1b/2a CONNECT SCI Study is ongoing, alongside preparation for a Phase 3 clinical trial in chronic SCI. NVG-291 has received Fast Track designation from the FDA and Orphan Drug designation from the European Medicines Agencyand follow NervGen on Xand LinkedIn.
Contacts
Huitt Tracey, Investors
htracey@nervgen.com
604.537.2094
David Schull or Ignacio Guerrero-Ros, Ph.D., Media
Russo Partners
David.Schull@russopartnersllc.com
Ignacio.Guerrero-Ros@russopartnersllc.com
858.717.2310
Bill Adams, Chief Financial Officer
info@nervgen.com
778.731.1711
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Cautionary Note and Forward Looking-Statements
This news release may contain "forward-looking information" and "forward-looking statements" within the meaning of applicable securities lawsand in NervGen's Form F-10/A filed on EDGAR at www.edgar.com. All clinical development plans are subject to additional funding. Readers should not place undue reliance on forward-looking statements made in this news release. Furthermore, unless otherwise stated, the forward-looking statements contained in this news release are made as of the date of this news release, and the Company has no intention and undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law. The forward-looking statements contained in this news release are expressly qualified by this cautionary statement.