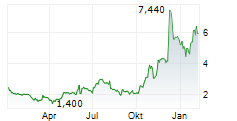
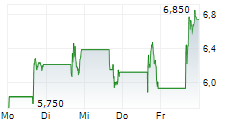
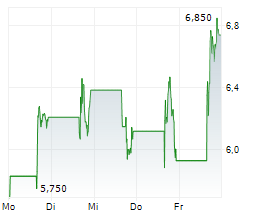
Immix Biopharma recently reported a positive clinical update for the registrational NEXICART-2 trial, evaluating lead CAR-T candidate NXC-201 in relapsed/refractory amyloid light chain amyloidosis (r/r ALA). We believe that both the safety and efficacy data offer promise to this fragile patient population, which has a true unmet medical need with no FDA-approved drugs in the r/r setting. The trial is expected to report a final readout in mid-2026, potentially representing another upcoming catalyst for Immix. Provided the results continue to be supportive, management intends to submit a biologics licence application (BLA) to the FDA before end-2026. In parallel with the clinical update, Immix also announced a $100m fund-raise, which management believes will provide a runway to mid-2027, beyond the conclusion of the lead NEXICART-2 programme. In light of these events, we have refreshed our valuation for Immix. We now value the company at $373.2m or $7.2 per share ($130.9m or $3.9 per share previously).Den vollständigen Artikel lesen ...
© 2026 Edison Investment Research