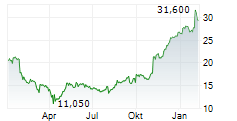
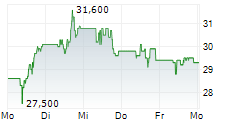
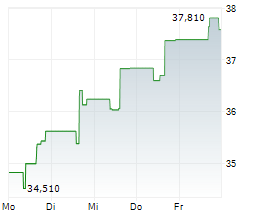
PETAH TIKVA (dpa-AFX) - Royalty Pharma plc (RPRX) and Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (TEVA), have entered into a funding agreement of up to $500 million to accelerate the clinical development of Teva's anti-IL-15 antibody, TEV-408. IL-15 is a key cytokine involved in multiple immune-mediated disease pathways. Early Phase 1b data from Teva's ongoing vitiligo study suggests IL-15 could be a promising therapeutic target for a broad range of autoimmune conditions. Teva expects to share trial results in 2026.
Under the terms of the agreement, Royalty Pharma will provide Teva with up to $500 million to support ongoing development costs for TEV-408 in vitiligo. This includes $75 million in R&D co-funding to initiate a Phase 2b study, which is planned to begin in 2026. Depending on the outcomes of the Phase 2b trial, Royalty Pharma will have the option to contribute an additional $425 million to co-fund the Phase 3 development program.
If TEV-408 is successfully approved and launched, Teva will pay Royalty Pharma a milestone payment along with royalties on worldwide net sales of the therapy.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News