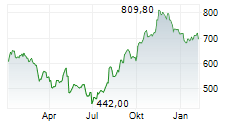
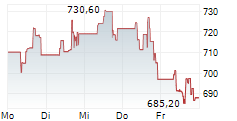
BRUSSELS (dpa-AFX) - argenx SE on Tuesday said the U.S. Food and Drug Administration has accepted for priority review a supplemental Biologics License Application (sBLA) for Vyvgart for the treatment of adults with acetylcholine receptor antibody-seronegative generalized myasthenia gravis.
The application has been granted a Prescription Drug User Fee Act target action date of May 10, 2026.
The sBLA is supported by data from the Phase 3 ADAPT SERON study evaluating Vyvgart in adults with AChR antibody-seronegative generalized myasthenia gravis.
Vyvgart is currently approved in the United States, the European Union, China and Canada for the treatment of adults with generalized myasthenia gravis who are anti-acetylcholine receptor antibody positive. In Japan, the drug is approved for adults with gMG who have shown an inadequate response to steroids or non-steroidal immunosuppressive therapies.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News