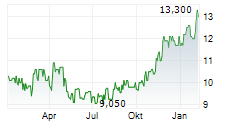
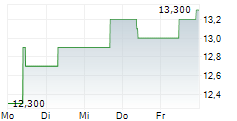
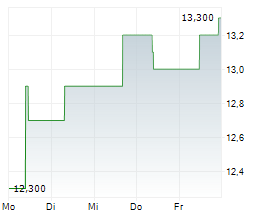
BRUSSELS/FRANKFURT/PARIS (dpa-AFX) - Ono Pharmaceutical Co. announced that Ono Pharma Taiwan Co., a Taiwanese subsidiary of Ono, received the additional approval of Opdivo Intravenous Infusion, an anti-PD-1 antibody, on January 14 from the Taiwan Food and Drug Administration in Taiwan, in combination with ipilimumab, for the treatment of adult patients with unresectable or metastatic microsatellite instability-high or mismatch repair deficient colorectal cancer. The approval is based on the results from the CheckMate-8HW study.
Opdivo as a single agent, or in combination with Yervoy, was previously granted accelerated approval in MSI-High/dMMR CRC adult patients that has progressed following treatment with a fluoropyrimidine, oxaliplatin and irinotecan. The company noted that the TFDA decision converts this second-line indication to full approval for Opdivo monotherapy, and expands the indication for Opdivo plus Yervoy into the first-line setting based on the CheckMate-8HW study.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News