PETAH TIKVA (dpa-AFX) - Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (TEVA), announced that the New England Journal of Medicine (NEJM) has published pivotal Phase 3 data on AJOVY, underscoring its efficacy and safety in children and adolescents with episodic migraine. The findings from the SPACE study supported the U.S. Food and Drug Administration (FDA) approval of AJOVY for the preventive treatment of episodic migraine in pediatric patients aged 6-17 years who weigh at least 45 kilograms (99 pounds).
AJOVY is now the first and only calcitonin gene-related peptide (CGRP) antagonist treatment option for the preventive treatment of episodic migraine in pediatric patients, as well as migraine in adults.
Results from the SPACE trial showed that AJOVY reduced monthly migraine days by 2.5 days compared to 1.4 days with placebo. It also reduced monthly headache days of at least moderate severity by 2.6 days versus 1.5 days with placebo. Importantly, a significantly higher proportion of participants achieved a - 50% reduction in monthly migraine days with AJOVY (47.2%) compared to placebo (27.0%). No new safety signals were identified, and the safety profile was consistent with that observed in adult studies.
The SPACE study was a 3-month, randomized, placebo-controlled trial that enrolled 237 children and adolescents aged 6-17 years with episodic migraine. Participants were randomized to receive monthly subcutaneous injections of fremanezumab-120 mg for those weighing less than 45 kg and 225 mg for those weighing 45 kg or more-or matched placebo.
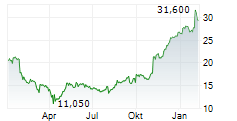
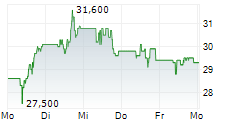
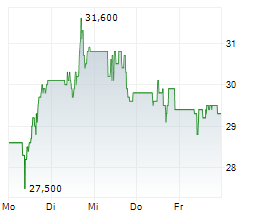
TEVA closed at $32.64, up $0.17 or 0.52% at the end of regular trading on Wednesday. In after-hours trading, the stock was slightly lower at $32.62, down $0.02 or 0.06% as of 8:20:21 PM EST.
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News