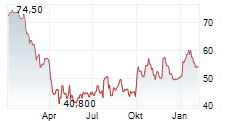
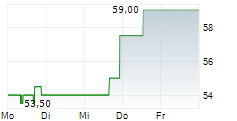
WASHINGTON (dpa-AFX) - Bio-Techne Corp. (TECH) said its Ella benchtop immunoassay platform has received CE-IVD marking and is now available for clinical use across the European Union.
The CE-IVD certification allows hospitals, clinical laboratories and diagnostic developers in Europe to use the Ella platform for in-house test development, clinical trials and other translational applications. The company said the milestone expands Ella's use beyond research settings into clinical environments.
Bio-Techne said the CE-IVD marking supports its long-term strategy to advance precision diagnostics and standardized biomarker detection.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News