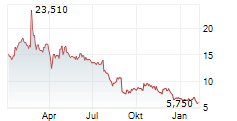
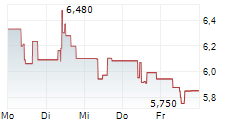
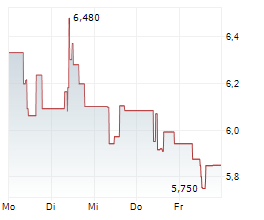
CANBERA (dpa-AFX) - Telix Pharmaceuticals Limited (TLX) has submitted a marketing authorization application in Europe for TLX101-Px, its glioma imaging candidate. The submission covers major European markets. The company noted that submission of the U.S. New Drug Application will follow.
Kevin Richardson, Chief Executive Officer, Telix Precision Medicine, said: 'We have been able to utilize aspects of our FDA package to expedite the European filing, which has been submitted in accordance with a pre-defined date agreed with the regulator, with the U.S. resubmission to follow.'
For More Such Health News, visit rttnews.com.
Copyright(c) 2026 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2026 AFX News