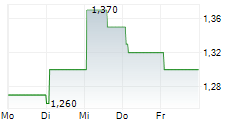
| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 1,230 | 1,360 | 12:45 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 11.02. | ABBISKO-B (02256): DATE OF BOARD MEETING | 1 | HKEx | ||
| 11.02. | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - FGFR4 INHIBITOR IRPAGRATINIB COMPLETED FIRST U.S. PATIENT DOSING IN THE EXPANSION PART OF A GLOBAL PHASE I ... | 1 | HKEx | ||
| ABBISKO CAYMAN Aktie jetzt für 0€ handeln | |||||
| 10.02. | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - FGFR4 INHIBITOR IRPAGRATINIB GRANTED FDA FAST TRACK DESIGNATION FOR HCC PATIENTS | 3 | HKEx | ||
| 13.01. | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - FDA ACCEPTANCE OF THE NDA FOR PIMICOTINIB FOR THE TREATMENT OF TGCT | 1 | HKEx | ||
| 13.01. | Abbisko Therapeutics Announces FDA Acceptance Of Pimicotinib NDA For Tenosynovial Giant Cell Tumor | 472 | AFX News | HONG KONG (dpa-AFX) - Abbisko Therapeutics Co., Ltd. announced that the U.S. Food and Drug Administration (FDA) has formally accepted the New Drug Application (NDA) for pimicotinib (ABSK021).... ► Artikel lesen | |
| 13.01. | Abbisko Therapeutics Announces FDA Acceptance of the NDA for Pimicotinib for the Treatment of Tenosynovial Giant Cell Tumor | 297 | PR Newswire | SHANGHAI, Jan. 12, 2026 /PRNewswire/ -- 13 January 2026 (Beijing Time), Abbisko Therapeutics Co., Ltd. ("Abbisko Therapeutics" hereafter, HKEX code: 02256.HK) announced... ► Artikel lesen | |
| 24.12.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - IND CLEARANCE FROM THE NMPA FOR ORAL SMALL MOLECULE KRAS G12D INHIBITOR ABSK141 | 5 | HKEx | ||
| 22.12.25 | Abbisko Therapeutics Co., Ltd.: Abbisko Therapeutics' CSF-1R Inhibitor Pimicotinib Approved by the China NMPA, Addressing the Treatment Gap for Tenosynovial Giant Cell Tumor | 282 | GlobeNewswire (Europe) | Based on the positive global Phase III MANEUVER study, the National Medical Products Administration (NMPA) has granted the world's first regulatory approval for pimicotinib, the first domestically... ► Artikel lesen | |
| 22.12.25 | ABBISKO-B (02256): INSIDE INFORMATION - ABBISKO THERAPEUTICS' CSF-1R INHIBITOR PIMICOTINIB APPROVED BY THE CHINA NMPA | 3 | HKEx | ||
| 16.12.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS COMPLETES FIRST PATIENT DOSING OF ABSK061, FGFR2/3 INHIBITOR, FOR THE TREATMENT OF ACHONDROPLASIA | 1 | HKEx | ||
| 08.12.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS PRESENTS PRELIMINARY PHASE II RESULTS OF ORAL PD-L1 INHIBITOR ABSK043 COMBINED WITH EGFR ... | 1 | HKEx | ||
| 01.12.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - IND CLEARANCE FROM THE FDA FOR ORAL SMALL MOLECULE KRAS G12D INHIBITOR ABSK141 | 1 | HKEx | ||
| 17.11.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS PRESENTS LONGER-TERM EFFICACY AND SAFETY OUTCOMES FROM PHASE III MANEUVER STUDY OF PIMICOTINIB ... | 1 | HKEx | ||
| 03.11.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS COMPLETES FIRST PATIENT DOSING IN A PHASE II STUDY OF ABSK043, AN ORAL SMALL-MOLECULE ... | 1 | HKEx | ||
| 27.10.25 | Goldman Sachs initiates Abbisko Therapeutics stock with Buy rating | 3 | Investing.com | ||
| 27.10.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS PRESENTED PRECLINICAL FINDINGS ON NOVEL CDK4/2 INHIBITOR AND SMARCA2 PROTAC DEGRADER ... | 1 | HKEx | ||
| 20.10.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS PRESENTS LONGER-TERM EFFICACY AND SAFETY OUTCOMES FROM PHASE III MANEUVER TRIAL OF PIMICOTINIB ... | 1 | HKEx | ||
| 29.09.25 | ABBISKO-B (02256): GRANT OF OPTIONS UNDER THE POST-IPO SHARE OPTION SCHEME AND GRANT OF RSUS UNDER THE 2019 SHARE INCENTIVE PLAN | 1 | HKEx | ||
| 15.09.25 | ABBISKO-B (02256): 2025 INTERIM REPORT | 3 | HKEx | ||
| 16.06.25 | Abbisko Therapeutics Completes First Patient Dosing in Registrational Study of Irpagratinib for HCC | 216 | PR Newswire | SHANGHAI, June 15, 2025 /PRNewswire/ -- Abbisko Therapeutics (HKEX Code: 02256) today announced that it has completed first patient dosing in a registrational... ► Artikel lesen |
1 2 Weiter >>
21 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| STRUCTURE THERAPEUTICS | 62,98 | 0,00 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| ARCELLX | 113,80 | -0,05 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| BIONTECH | 93,45 | +0,16 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| QIAGEN | 41,370 | -0,05 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 14,750 | 0,00 % | Novartis Pharma AG: Novartis successfully completes acquisition of Avidity Biosciences, strengthening late-stage neuroscience pipeline and advancing xRNA strategy | Adds Avidity's differentiated muscle-directed Antibody Oligonucleotide Conjugates (AOC) platform and three late-stage programs to industry-leading neuromuscular pipelinePotentially unlocks multi-billion-dollar... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | 0,00 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| CARDIO DIAGNOSTICS | 6,490 | 0,00 % | Women in power: Scatec CDIO Kine Årdal conquers oil and gas, renewables and digitalisation | ||
| ADMA BIOLOGICS | 15,580 | 0,00 % | ADMA Q4 EPS Up 14% Y/Y, Revenues Gain From Strong Asceniv Performance | ||
| RELAY THERAPEUTICS | 10,250 | 0,00 % | Relay Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| PRAXIS PRECISION MEDICINES | 336,75 | 0,00 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| VERA THERAPEUTICS | 40,820 | 0,00 % | Vera Therapeutics, Inc. - 10-K, Annual Report | ||
| KYMERA THERAPEUTICS | 91,36 | -3,85 % | Morgan Stanley cuts Kymera Therapeutics stock price target on pipeline progress | ||
| COGENT BIOSCIENCES | 38,870 | 0,00 % | Cogent Biosciences, Inc.: Cogent Biosciences Highlights Additional Data with Six Bezuclastinib Posters from SUMMIT Trial at 2026 AAAAI Annual Meeting | Bezuclastinib mean TSS reduction deepens to -32.0 points at 48 weeks of treatment with further improvement shown across all measured symptoms99% of patients achieve >50% reduction in serum tryptase... ► Artikel lesen | |
| EDGEWISE THERAPEUTICS | 30,450 | 0,00 % | Edgewise Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results with Strong Progress Across Muscular Dystrophy and Cardiovascular Programs | - CIRRUS-HCM 12-week data of EDG-7500 in obstructive and nonobstructive hypertrophic cardiomyopathy (HCM) expected in H1 2026 -
- Phase 1 healthy adult trial... ► Artikel lesen | |
| PRIME MEDICINE | 4,630 | 0,00 % | Prime Medicine, Inc.: Prime Medicine Reports Third Quarter 2025 Financial Results and Provides Business Updates | -- New preclinical data for PM577 in Wilson's Disease (WD) to be presented at AASLD; on-track to file IND and/or CTA in H1'26, with initial clinical data expected in 2027 -- -- Nominated PM647 as... ► Artikel lesen |
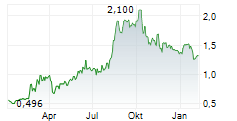
ABBISKO CAYMAN LTD gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell 0,00 % bei einem Aktienkurs von 1,300 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ABBISKO CAYMAN LTD finden Sie auf Wallstreet Online.