- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | Guggenheim stuft Cullinan Oncology mit "Kaufen" ein und sieht 151 % Kurspotenzial | 4 | Investing.com Deutsch | ||
| Mo | Guggenheim initiates Cullinan Oncology stock with Buy rating, $30 target | 9 | Investing.com | ||
| 08.01. | Cullinan Therapeutics: Wichtige Studiendaten zu Autoimmun- und Krebstherapien für 2026 geplant | 3 | Investing.com Deutsch | ||
| 08.01. | Cullinan Therapeutics plans data readouts for key autoimmune programs | 2 | Investing.com | ||
| 08.01. | Cullinan Therapeutics, Inc.: Cullinan Therapeutics Provides Corporate Update and Highlights Anticipated 2026 Milestones | 623 | GlobeNewswire (Europe) | Data readouts planned for CLN-978 across all three autoimmune indications in 2026, including single dose and repeat dosing data Company to complete monotherapy expansion cohorts to determine recommended... ► Artikel lesen | |
| 08.01. | Cullinan Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 17.12.25 | Cowen reiterates Buy rating on Cullinan Oncology stock after ASH data | 5 | Investing.com | ||
| CULLINAN THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 08.12.25 | Cullinan Therapeutics, Inc.: Cullinan Therapeutics Showcases Compelling Clinical Data in AML for CLN-049, Novel FLT3xCD3 T Cell Engager, in Oral Presentation at the 67th ASH Meeting | 15 | GlobeNewswire (USA) | ||
| 08.12.25 | Cullinan Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 01.12.25 | Cullinan Therapeutics Announces Fast Track Designation For CLN-049 | 2 | RTTNews | ||
| 01.12.25 | Cullinan Therapeutics, Inc.: Cullinan Therapeutics Receives FDA Fast Track Designation for CLN-049, a Novel FLT3xCD3 T Cell Engager, in Relapsed/Refractory Acute Myeloid Leukemia | 235 | GlobeNewswire (Europe) | Promising efficacy and favorable safety data from the Phase 1 study in heavily pre-treated patients show potential of CLN-049 to address a broad population of AML patients CAMBRIDGE, Mass., Dec. 01... ► Artikel lesen | |
| 26.11.25 | Cullinan Oncology stock price target raised to $36 at Jones Trading | 2 | Investing.com | ||
| 25.11.25 | Vielversprechende AML-Daten: BTIG erhöht Kursziel für Cullinan Oncology auf 38 US-Dollar | 12 | Investing.com Deutsch | ||
| 25.11.25 | Cullinan Oncology stock price target raised to $38 at BTIG on promising AML data | 2 | Investing.com | ||
| 21.11.25 | Cullinan Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 21.11.25 | H.C. Wainwright raises Cullinan Oncology stock price target on NDA progress | 4 | Investing.com | ||
| 21.11.25 | Fortschritte bei Zipalertinib-Zulassung: H.C. Wainwright hebt Kursziel für Cullinan Oncology an | 13 | Investing.com Deutsch | ||
| 20.11.25 | Cullinan Therapeutics rises on rolling submission for zipalertinib | 1 | Seeking Alpha | ||
| 06.11.25 | Cullinan Therapeutics GAAP EPS of -$0.77 | 3 | Seeking Alpha | ||
| 06.11.25 | Cullinan Therapeutics, Inc. - 10-Q, Quarterly Report | - | SEC Filings |
1 2 Weiter >>
34 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIOCRYST PHARMACEUTICALS | 5,392 | 0,00 % | BioCryst Pharmaceuticals, Inc.: BioCryst to Report Fourth Quarter 2025 Financial Results on February 26 | ||
| MICROBOT MEDICAL | 1,475 | -1,80 % | Microbot Medical Inc. - 8-K, Current Report | ||
| IMMUNITYBIO | 5,118 | +0,08 % | ImmunityBio launches phase 2 trial for chemotherapy-free lymphoma therapy | ||
| VOYAGER THERAPEUTICS | 3,126 | +0,51 % | Voyager Therapeutics, Inc.: Voyager Reports Third Quarter 2025 Financial and Operating Results | - Momentum building around tau: expect VY7523 clinical data and VY1706 clinical entry in 2026 - - Sharpened focus on multi-modality pipeline with introduction of Voyager NeuroShuttle discovery... ► Artikel lesen | |
| CAPRICOR | 19,000 | -9,31 % | Capricor Therapeutics Provides Regulatory Update on Deramiocel BLA Following FDA Review of HOPE-3 Topline Data | FDA has requested the HOPE-3 clinical study report (CSR) as part of the BLA review processCompany expects to submit updates to the BLA in February 2026 to support continued FDA review SAN DIEGO,... ► Artikel lesen | |
| ABEONA THERAPEUTICS | 4,280 | -1,38 % | Abeona Therapeutics Inc.: Abeona Therapeutics Announces Appointment of Mohamad Tabrizi as Chief Business Officer | CLEVELAND, Dec. 15, 2025 (GLOBE NEWSWIRE) -- Abeona Therapeutics Inc. (Nasdaq: ABEO) today announced the appointment of Mohamad Tabrizi, M.S., M.B.A., as Senior Vice President, Chief Business Officer... ► Artikel lesen | |
| ZEVRA THERAPEUTICS | 7,200 | -1,37 % | Zevra Therapeutics to Ring Nasdaq Stock Market Opening Bell on Monday, February 9, 2026 | ||
| VIR BIOTECHNOLOGY | 7,180 | +3,76 % | EQS-News: Norgine Pharmaceuticals Limited: Norgine erweitert sein Hepatologie- und Spezialitätenportfolio durch exklusive Einlizenzierungsvereinbarung mit Vir Biotechnology | EQS-News: Norgine Pharmaceuticals Limited
/ Schlagwort(e): Produkteinführung/Vereinbarung
Norgine erweitert sein Hepatologie- und Spezialitätenportfolio durch exklusive Einlizenzierungsvereinbarung... ► Artikel lesen | |
| PALVELLA THERAPEUTICS | 65,00 | -7,14 % | Palvella Therapeutics: Clear Street bestätigt Kaufempfehlung im Vorfeld von Studiendaten | ||
| KALVISTA PHARMACEUTICALS | 12,200 | -3,17 % | KalVista Pharmaceuticals, Inc.: KalVista Pharmaceuticals Reports Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | KalVista Pharmaceuticals, Inc. (Nasdaq: KALV), today announced that the Compensation Committee of KalVista's Board of Directors granted six newly-hired employees inducement options to purchase an... ► Artikel lesen | |
| DBV TECHNOLOGIES | 3,520 | -3,43 % | DBV Technologies S.A.: Information Regarding the Total Number of Voting Rights and Total Number of Shares of the Company as of January 31, 2026 | Information Regarding the Total Number of Voting Rights and Total Number of Shares of the Company as of January 31, 2026
(Article 223-16 of the General Regulations of the Autorité des Marchés Financiers)
Market... ► Artikel lesen | |
| MARKER THERAPEUTICS | 1,260 | -9,35 % | Marker Therapeutics Reports Third Quarter 2025 Financial Results and Provides Business Updates | APOLLO study showed encouraging overall responses and favorable safety profile in relapsed/refractory B-cell lymphoma MT-601 demonstrated 66% objective response rate with 50% complete response in... ► Artikel lesen | |
| CARTESIAN THERAPEUTICS | 5,500 | +0,92 % | Cartesian Therapeutics, Inc.: Cartesian Therapeutics Announces New Employment Inducement Grants | ||
| ALIGOS THERAPEUTICS | 5,400 | -12,90 % | Aligos Therapeutics, Inc. - 8-K, Current Report | ||
| QUANTUM-SI | 1,060 | +13,72 % | Quantum-Si Inc - 8-K, Current Report |
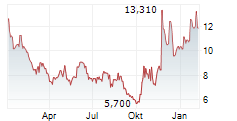
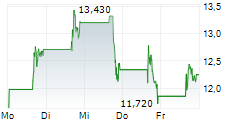
CULLINAN THERAPEUTICS INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 12,250 Euro und damit +3,38 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CULLINAN THERAPEUTICS INC finden Sie auf Wallstreet Online.