- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 06.02. | HUTCHMED Ltd - 6-K, Report of foreign issuer | 2 | SEC Filings | ||
| 06.02. | HUTCHMED gibt Jahresergebnisse 2025 am 5. März bekannt | 1 | Investing.com Deutsch | ||
| 06.02. | HUTCHMED to announce 2025 final results on March 5 | 1 | Investing.com | ||
| 06.02. | HUTCHMED (China) Limited: HUTCHMED to Announce 2025 Final Results | 1 | GlobeNewswire (USA) | ||
| 06.02. | HUTCHMED (00013): DATE OF BOARD MEETING AND ANNOUNCEMENT OF 2025 FINAL RESULTS | 2 | HKEx | ||
| HUTCHMED Aktie jetzt für 0€ handeln | |||||
| 06.02. | Hutchmed China Ltd - HUTCHMED to Announce 2025 Final Results | - | RNS | ||
| 14.01. | Hutchmed China hails lung cancer trial results published in Lancet | 4 | Alliance News | ||
| 14.01. | HUTCHMED Ltd - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 14.01. | Hutchmed China Ltd - Publication of Phase III Results in The Lancet | - | RNS | ||
| 14.01. | HUTCHMED Announces Publication Of Phase III SACHI Study Results In The Lancet | 384 | AFX News | LONDON (dpa-AFX) - HUTCHMED (China) Limited (HCM) on Wednesday said results from the Phase III SACHI trial evaluating savolitinib in combination with osimertinib were published in The Lancet.The... ► Artikel lesen | |
| 14.01. | HUTCHMED (China) Limited: HUTCHMED Highlights Publication of Phase III SACHI Results in The Lancet | 4 | GlobeNewswire (USA) | ||
| 14.01. | HUTCHMED (00013): VOLUNTARY ANNOUNCEMENT - HUTCHMED HIGHLIGHTS PUBLICATION OF PHASE III SACHI RESULTS IN THE LANCET | 2 | HKEx | ||
| 07.01. | Hutchmed scores with drug for rare autoimmune disease | - | pharmaphorum | ||
| 07.01. | HUTCHMED-Aktie legt zu: Sovleplenib erreicht primären Endpunkt in Phase-III-Studie | 3 | Investing.com Deutsch | ||
| 07.01. | Positive China Trial Data Moves HUTCHMED Closer To Rare Blood Disorder Drug Filing In 2026 | 1 | Benzinga.com | ||
| 07.01. | Hutchmed China hails sovleplenib results for form of anaemia in China | 1 | Alliance News | ||
| 07.01. | Hutchmed heats up autoimmune race with phase 3 win, teeing up China filing | 2 | FierceBiotech | ||
| 07.01. | Hutchmed's sovleplenib meets primary endpoint in wAIHA trial | 1 | Investing.com | ||
| 07.01. | Hutchmed China Ltd - Positive Topline Results of Phase III Trial | - | RNS | ||
| 07.01. | HUTCHMED's Sovleplenib Reaches Phase III Goal In Warm Autoimmune Hemolytic Anemia Trial In China | 363 | AFX News | LONDON (dpa-AFX) - HUTCHMED (China) Limited (HCM, HCM.L, 0013.HK) announced that the Phase III registration portion of the ESLIM-02 clinical trial evaluating sovleplenib, a novel spleen tyrosine... ► Artikel lesen |
1 2 3 4 5 Weiter >>
124 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 42,010 | +0,39 % | Bayer Aktie: Alles vorbei? - Airbus, BB Biotech, BioNTech, TUI und Valneva - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| NOVO NORDISK | 31,600 | -0,24 % | Novo Nordisk enttäuscht mit Abnehmspritze CagriSema - Eli Lillys Präparat überlegen | Der dänische Pharmakonzern Novo Nordisk hat mit seinem Kombipräparat CagriSema das primäre Ziel der Phase-3-Studie REDEFINE 4 verfehlt. Das Medikament erreichte nach 84 Wochen zwar einen Gewichtsverlust... ► Artikel lesen | |
| PFIZER | 23,350 | -0,17 % | Pfizer, Astellas Pharma Share Positive Data From Bladder Cancer Trial | NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and Astellas Pharma Inc., Friday announced the positive results from the Phase 3 EV-304 clinical trial for PADCEV in combination with Keytruda in... ► Artikel lesen | |
| MERCK KGAA | 128,60 | +2,35 % | Big Blue IBM, Fastly & Merck KGaA - Was sagen Charts UND Fundamentaldaten wirklich? | ||
| GILEAD SCIENCES | 125,66 | -0,33 % | Gilead Sciences auf Einkaufstour: Wer folgt als Nächstes? | Gilead Sciences lässt seinen Worten Taten folgen: Mit einer ersten Multi-Milliarden-Übernahme im laufenden Jahr stärkt der Pharmakonzern seine Pipeline. Weiterhin ausstehend: eine bedeutende Transaktion... ► Artikel lesen | |
| AURORA CANNABIS | 3,240 | -1,22 % | Is Aurora Cannabis Stock Ready to 10X in the Next Pot Boom? | ||
| GSK | 25,040 | +0,93 % | JPMORGAN stuft GSK auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für GSK nach einer Telefonkonferenz auf "Underweight" mit einem Kursziel von 1700 Pence belassen. Mit einer Übernahme von 35Pharma... ► Artikel lesen | |
| ABBVIE | 196,00 | -0,31 % | Rekordquartal und AbbVie-Deal beflügeln Gubra-Aktie: Kursplus von 13,88 % | ||
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| MERCK & CO | 104,60 | -0,19 % | Pharma im Umbruch: Milliardenblockbuster vor Patentende: Merck baut Konzern radikal um | © Foto: JHVEPhoto - stock.adobe.com Merck stellt sein Pharmageschäft neu auf. Der Konzern reagiert auf das nahende Patentende seines Kassenschlagers Keytruda und setzt auf neue Wachstumstreiber.Der... ► Artikel lesen | |
| ASTRAZENECA | 177,25 | +0,45 % | UBS stuft ASTRAZENECA auf 'Buy' | ZÜRICH (dpa-AFX Analyser) - Die Schweizer Großbank UBS hat das Kursziel für Astrazeneca von 16300 auf 17600 Pence angehoben und die Einstufung auf "Buy" belassen. Astrazeneca stehe zum Ende dieses Jahrzehnts... ► Artikel lesen | |
| TILRAY BRANDS | 6,670 | -0,15 % | MDMA-Herausforderer Bioxyne punktet mit 112% Umsatzplus. Und was macht Tilray? | ||
| BRISTOL-MYERS SQUIBB | 52,90 | +0,21 % | Bristol Myers Squibb: Setzt sich die relative Stärke der Pharmaaktien fort? | Nach der jüngsten Aufwärtsbewegung zeigt die Aktie von Bristol Myers Squibb eine Konsolidierung. Das Chartbild wirkt dabei weiterhin stabil Den vollständigen Artikel lesen ... ► Artikel lesen | |
| TEVA | 28,100 | -2,09 % | Teva Pharmaceutical Industries Ltd: Teva to Present at the Upcoming Investor Conferences in March | PARSIPPANY, N.J. and TEL AVIV, Israel, Feb. 24, 2026 (GLOBE NEWSWIRE) -- Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) today announced that Richard Francis, Teva's President and CEO, will... ► Artikel lesen | |
| ABBOTT LABORATORIES | 98,07 | -0,38 % | Exact Sciences (EXAS) Merger with Abbott Moving Forward After Shareholder Approval |
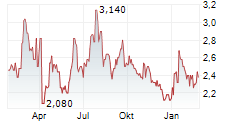
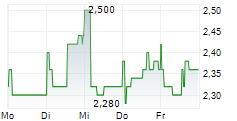
Das Unternehmen HUTCHMED CHINA LIMITED kann der Branche Pharma zugeordnet werden. Der aktuelle Kurs liegt bei 2,360 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu HUTCHMED CHINA LIMITED finden Sie auf Wallstreet Online.