- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 11.02. | Nuvectis Pharma GAAP EPS of -$1.32 | 1 | Seeking Alpha | ||
| 11.02. | Nuvectis Pharma, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 11.02. | Nuvectis Pharma, Inc. - 10-K, Annual Report | 1 | SEC Filings | ||
| 11.02. | Nuvectis Pharma, Inc. Reports 2025 Financial Results and Business Highlights | 127 | GlobeNewswire (Europe) | FORT LEE, N.J., Feb. 11, 2026 (GLOBE NEWSWIRE) -- Nuvectis Pharma, Inc. (NASDAQ: NVCT) ("Nuvectis" or the "Company"), a clinical-stage biopharmaceutical company focused on the development of innovative... ► Artikel lesen | |
| 17.12.25 | Nuvectis startet Phase-1b-Studie zur Kombination von NXP900 mit Osimertinib bei Lungenkrebs | 2 | Investing.com Deutsch | ||
| NUVECTIS PHARMA Aktie jetzt für 0€ handeln | |||||
| 17.12.25 | Nuvectis Pharma, Inc.: Nuvectis Pharma Announces the Initiation of the Phase 1b Study of NXP900 in Combination with Osimertinib in Patients with NSCLC | 152 | GlobeNewswire (Europe) | Fort Lee, NJ, Dec. 17, 2025 (GLOBE NEWSWIRE) -- Nuvectis Pharma, Inc. (NASDAQ: NVCT), a clinical stage biopharmaceutical company focused on the development of innovative precision medicines for the... ► Artikel lesen | |
| 03.12.25 | Nuvectis Pharma stock rating reiterated at Buy by H.C. Wainwright | 1 | Investing.com | ||
| 25.11.25 | Nuvectis Pharma, Inc.: Nuvectis Pharma to Host a Virtual Key Opinion Leader Meeting to Discuss the NXP900 Phase 1b Program in Advanced Solid Tumors, Including the Combination with Osimertinib in NSCLC | 2 | GlobeNewswire (USA) | ||
| 04.11.25 | Nuvectis Pharma reports Q3 results | 1 | Seeking Alpha | ||
| 04.11.25 | Nuvectis Pharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 04.11.25 | Nuvectis Pharma, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 28.10.25 | H.C. Wainwright bestätigt "Buy"-Rating für Nuvectis Pharma mit Kursziel von 10 US-Dollar | 16 | Investing.com Deutsch | ||
| 28.10.25 | H.C. Wainwright reiterates Buy rating on Nuvectis Pharma stock at $10 target | 1 | Investing.com | ||
| 25.09.25 | Nuvectis Pharma, Inc. - 8-K, Current Report | 3 | SEC Filings | ||
| 11.08.25 | Nuvectis Pharma, Inc.: Nuvectis Pharma Announces the Initiation of the Phase 1b Program for NXP900 | 523 | GlobeNewswire (Europe) | The Phase 1b program is designed to evaluate the clinical activity of NXP900 as a single agent in patients with advanced solid tumors whose cancers harbor specific genetic alterations, and in combination... ► Artikel lesen | |
| 05.08.25 | Nuvectis Pharma, Inc. Reports Second Quarter 2025 Financial Results and Business Highlights | 364 | GlobeNewswire (Europe) | NXP900 becomes the lead drug candidate after successfully completing the Phase 1a dose escalation study in patients with advanced solid tumors and a drug-drug interaction study in healthy volunteers... ► Artikel lesen | |
| 31.07.25 | Nuvectis Pharma, Inc.: Nuvectis Pharma Provides Final Clinical Data Update from the NXP800 Phase 1b Study in Ovarian Cancer and Reports Completion of the NXP900 Phase 1a Dose Escalation Study | 215 | GlobeNewswire (Europe) | Available data from 13 patients with recurrent, platinum resistant, ARID1a-mutated ovarian cancer treated with 75 mg/day in the NXP800 Phase 1b study includes 2 partial responses and 3 stable diseases... ► Artikel lesen | |
| 06.05.25 | Nuvectis Pharma, Inc. Reports First Quarter 2025 Financial Results and Business Highlights | 194 | GlobeNewswire (Europe) | NXP900 clinical data presentation from the Phase 1a dose escalation study at the 2025 American Association for Cancer Research (AACR) conference demonstrated robust pharmacodynamic response and acceptable... ► Artikel lesen |
18 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| STRUCTURE THERAPEUTICS | 62,98 | 0,00 % | Structure Therapeutics Inc.: Structure Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results and Recent Highlights | Positive results from the aleniglipron Phase 2 ACCESS programs in December 2025 demonstrated significant weight loss across all doses and up to 15.3% at 36 weeks Topline 44-week data from the ACCESS... ► Artikel lesen | |
| ARCELLX | 113,80 | -0,05 % | Aktien New York Ausblick: Leichte Verluste nach Trumps Zollankündigung | NEW YORK (dpa-AFX) - Die US-Börsen steuern am Montag vor dem Hintergrund neuer Zollunsicherheit auf einen etwas tieferen Handelsauftakt zu. Nachdem der Oberste Gerichtshof der USA dem Präsidenten Donald... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 14,750 | 0,00 % | Novartis Pharma AG: Novartis successfully completes acquisition of Avidity Biosciences, strengthening late-stage neuroscience pipeline and advancing xRNA strategy | Adds Avidity's differentiated muscle-directed Antibody Oligonucleotide Conjugates (AOC) platform and three late-stage programs to industry-leading neuromuscular pipelinePotentially unlocks multi-billion-dollar... ► Artikel lesen | |
| VIR BIOTECHNOLOGY | 9,100 | 0,00 % | Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen | |
| CARDIO DIAGNOSTICS | 6,490 | 0,00 % | Women in power: Scatec CDIO Kine Årdal conquers oil and gas, renewables and digitalisation | ||
| BIONTECH | 92,50 | -0,86 % | Aufgrund von angeblichen Patentverletzungen zieht BioNTech gegen Moderna vor Gericht | Die Hersteller von Corona-Impfstoffen haben sich schon seit Jahren in den Haaren. Immer wieder gibt es Vorwürfe über Patentverletzungen. So auch in einem aktuellen Fall, bei dem BioNTech gegen den US-Hersteller... ► Artikel lesen | |
| ADMA BIOLOGICS | 15,580 | 0,00 % | ADMA Q4 EPS Up 14% Y/Y, Revenues Gain From Strong Asceniv Performance | ||
| RELAY THERAPEUTICS | 10,250 | 0,00 % | Relay Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | ||
| PRAXIS PRECISION MEDICINES | 336,75 | -1,05 % | Praxis Precision Medicines, Inc.: Praxis Precision Medicines Provides Corporate Update and Reports Fourth Quarter and Full-Year 2025 Financial Results | Two new drug applications (NDA) for ulixacaltamide in essential tremor (ET) and for relutrigine in SCN2A and SCN8A developmental and epileptic encephalopathies (DEEs) have been submitted to the U.S.... ► Artikel lesen | |
| VERA THERAPEUTICS | 40,820 | 0,00 % | Vera Therapeutics, Inc. - 10-K, Annual Report | ||
| QIAGEN | 41,290 | -0,24 % | flatexDEGIRO, Qiagen, Börse München - 4investors-Weekend | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Und welche Titel haben besonders starke Kursbewegungen verzeichnet? Vor allem für Trader ist es wichtig zu wissen... ► Artikel lesen | |
| KYMERA THERAPEUTICS | 91,36 | -3,85 % | Morgan Stanley cuts Kymera Therapeutics stock price target on pipeline progress | ||
| COGENT BIOSCIENCES | 38,870 | 0,00 % | Cogent Biosciences, Inc.: Cogent Biosciences Highlights Additional Data with Six Bezuclastinib Posters from SUMMIT Trial at 2026 AAAAI Annual Meeting | Bezuclastinib mean TSS reduction deepens to -32.0 points at 48 weeks of treatment with further improvement shown across all measured symptoms99% of patients achieve >50% reduction in serum tryptase... ► Artikel lesen | |
| EDGEWISE THERAPEUTICS | 30,450 | 0,00 % | Edgewise Therapeutics Reports Fourth Quarter and Full Year 2025 Financial Results with Strong Progress Across Muscular Dystrophy and Cardiovascular Programs | - CIRRUS-HCM 12-week data of EDG-7500 in obstructive and nonobstructive hypertrophic cardiomyopathy (HCM) expected in H1 2026 -
- Phase 1 healthy adult trial... ► Artikel lesen | |
| PRIME MEDICINE | 4,630 | 0,00 % | Prime Medicine, Inc.: Prime Medicine Reports Third Quarter 2025 Financial Results and Provides Business Updates | -- New preclinical data for PM577 in Wilson's Disease (WD) to be presented at AASLD; on-track to file IND and/or CTA in H1'26, with initial clinical data expected in 2027 -- -- Nominated PM647 as... ► Artikel lesen |
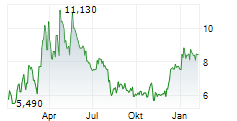
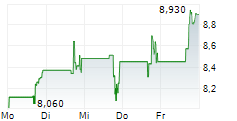
Das Unternehmen NUVECTIS PHARMA INC kann der Branche Biotechnologie zugeordnet werden. Mit einer aktuellen Kursveränderung von -0,025 (-0,28 %) liegt der Kurs bei 8,895 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu NUVECTIS PHARMA INC finden Sie auf Wallstreet Online.