Chart-Typ:
Zeitraum:
| PTA-NVR: QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG | DJ PTA-NVR: QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG
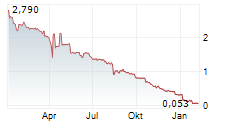
Gesamtstimmrechte gemäß -- 41 WpHG
QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach... ► Artikel lesen |
| Shortseller-Positionen aktuell: Auto1, Energiekontor, freenet, Gerresheimer, Puma, Qiagen, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
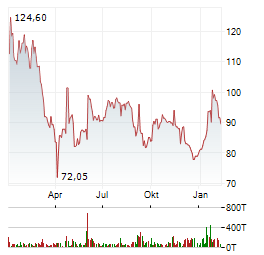
| 5 Milliarden Dollar verbrannt: BioNTech nach Gründer-Crash: Aktie plötzlich spottbillig? | © Foto: Chris Emil Janssen - chris.emil-janssen.deDer Rückzug der mRNA-Pioniere ließ Milliarden an Börsenwert verdampfen. Einige Analysten halten die BioNTech-Aktie nach dem Schock aber plötzlich für... ► Artikel lesen |
| BioNTech Aktie: Kursziele gekappt - Deutsche Bank, Gerresheimer, Rheinmetall, Smartbroker und VW - Börse Frankfurt | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen |
| PRESSESPIEGEL/Unternehmen: BIONTECH, DEUTSCHE BAHN, SIGNA, EDEKA/TEGUT, BLOOMBERG, REVOLUT, MCKINSEY, PSI SOFTWARE | DJ PRESSESPIEGEL/Unternehmen
Die wirtschaftsrelevanten Themen aus den Medien, zusammengestellt von Dow Jones Newswires.
BIONTECH - Biontech wird an dem neuen Unternehmen, das die Gründer... ► Artikel lesen |
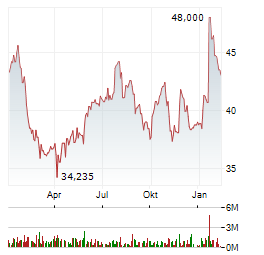
| Evotec: Eine klare Enttäuschung | Die Analysten der Deutschen Bank bezeichnen das Strategie-Update von Evotec als enttäuschend. 2026 sollen die Umsätze noch stärker als 2025 zurückgehen. Das bereinigte EBITDA soll sich auf rund 20 Millionen... ► Artikel lesen |
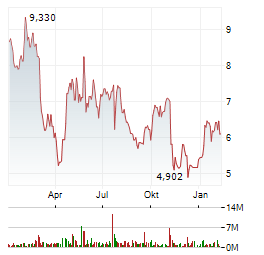
| PAUKENSCHLAG! ÜBERNAHMEFANTASIE? BioNTech, Evotec, Vidac Pharma | Zunächst zum Positiven: Der Wirkstoff VDA-1102 von Vidac Pharma wurde im Rahmen einer Härtefallregelung eingesetzt. Und zwar rund um die dritte Gehirnoperation eines Mädchens. Der Patientin ging es... ► Artikel lesen |
| Evotec-Aktie fällt und fällt - tiefster Stand seit 2016! | Der Hamburger Wirkstoffforscher Evotec hat bereits am Dienstag mit Eckdaten für das vergangene Geschäftsjahr 2025 sowie einem Ausblick auf 2026 und der Ankündigung weiterer Sparmaßnahmen für eine herbe... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu AQUAPORIN A/S finden Sie auf Wallstreet Online.