| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
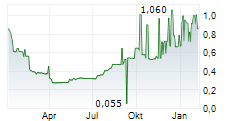
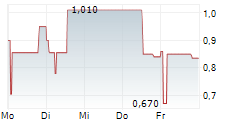
| 0,790 | 0,850 | 08:29 |
Chart-Typ:
Zeitraum:
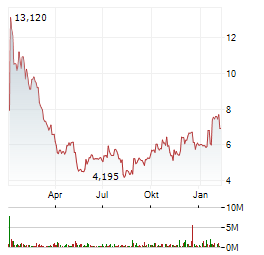
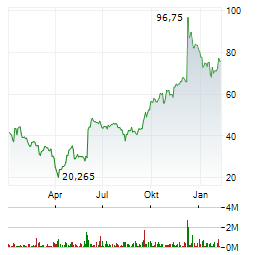
| Vir Biotechnology +70%: Deal-Überraschung mit Ansage | Die Aktie von Vir Biotechnology ist vorbörslich um rund 70 Prozent nach oben geschossen. Auslöser der Kursexplosion ist vor allem ein milliardenschwerer Kooperationsdeal mit dem japanischen Pharmakonzern... ► Artikel lesen |
| Vir Biotechnology, Inc.: Vir Biotechnology Reports Positive Updated Phase 1 Results for PSMA-targeting, PRO-XTEN Dual-masked T-Cell Engager VIR-5500 in Patients with Metastatic Prostate Cancer | Updated Phase 1 dose-escalation data (n=58) show VIR-5500 monotherapy has a favorable safety profile and was well tolerated with no dose-limiting toxicities observed to date
- Dose-dependent anti-tumor... ► Artikel lesen |
| Astellas Pharma Inc.: Astellas and Vir Biotechnology Announce Global Strategic Collaboration to Advance PSMA-targeting PRO-XTEN Dual-masked T-Cell Engager VIR-5500 for the Treatment of Prostate Cancer | - Astellas and Vir Biotechnology to co-develop and co-commercialize VIR-5500 through a sharing of expenses and revenues -- Astellas to lead commercialization of VIR-5500 in the U.S. with... ► Artikel lesen |
| Weekly Buzz: MGNX's LINNET Trial On Hold; Eton Pharmaceuticals, ALUR Get FDA Nod; GILD Snaps Up ACLX | FOSTER CITY (dpa-AFX) - This week, the biotech space witnessed significant milestones, including FDA approvals, oncology drug acquisitions, and collaborations. Positive clinical trial results... ► Artikel lesen |
| ROUNDUP/Aktien New York Schluss: Klare Verluste - KI-Ängste und Zölle belasten | NEW YORK (dpa-AFX) - Die US-Aktienmärkte haben einen Fehlstart in die neue Börsenwoche hingelegt. Erneute Sorgen um die Auswirkungen Künstlicher Intelligenz (KI) auf die Unternehmensgewinne sowie die... ► Artikel lesen |
| Aktien New York: Schwacher Wochenstart - KI-Ängste und Zölle belasten | NEW YORK (dpa-AFX) - Die US-Aktienmärkte sind mit klaren Verlusten in die neue Börsenwoche gestartet. Erneute Sorgen um die Auswirkungen Künstlicher Intelligenz (KI) auf die Unternehmensgewinne sowie... ► Artikel lesen |
| Kymera Therapeutics, Inc.: Kymera Therapeutics Announces First Patient Dosed in BREADTH Phase 2b Asthma Clinical Trial of KT-621, a First-in-Class, Oral STAT6 Degrader | Data from the BREADTH Phase 2b asthma trial is expected to be reported in late-2027 Data from the ongoing parallel BROADEN2 Phase 2b atopic dermatitis trial is expected to be reported by mid-2027... ► Artikel lesen |
| Kymera Therapeutics, Inc.: Kymera Therapeutics Outlines Key 2026 Objectives and Strategy to Advance Industry Leading Portfolio of Oral Immunology Programs | KT-621 BROADEN2 Phase 2b trial in AD ongoing, with data expected by mid-2027 KT-621 BREADTH Phase 2b trial in asthma initiated, with data expected in late-2027 KT-579 Phase 1 HV clinical trial expected... ► Artikel lesen |
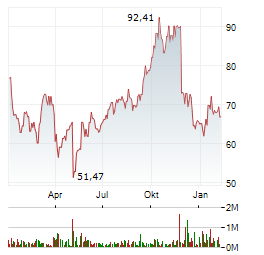
| Kymera Therapeutics, Inc.: Kymera Therapeutics Announces Closing of Upsized $602 Million Public Offering and Full Exercise of Underwriters' Option to Purchase Additional Shares | WATERTOWN, Mass., Dec. 11, 2025 (GLOBE NEWSWIRE) -- Kymera Therapeutics, Inc. (NASDAQ: KYMR), a clinical-stage biopharmaceutical company advancing a new class of oral small molecule degrader medicines... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ARECOR THERAPEUTICS PLC finden Sie auf Wallstreet Online.