| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
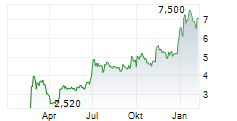
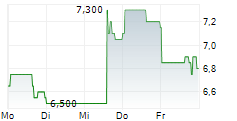
| 6,850 | 7,500 | 13:29 |
Chart-Typ:
Zeitraum:
| Befreiungsschlag bei Plug Power? Neuer CEO verspricht das Ende der roten Zahlen! | Lange Zeit war es ruhig um den einstigen Highflyer Plug Power - doch am Dienstagmittag meldet sich der US-Konzern mit einem Paukenschlag zurück. Trotz eines dicken Minus in der Bilanz schicken die Anleger... ► Artikel lesen |
| Plug Power News: zweistelliges Umsatzplus, Bruttogewinn und neuer CEO | LATHAM, New York (IT-Times) - Plug Power, Anbieter von Wasserstoff- und Brennstoffzellen-Lösungen, hat seine Ergebnisse für das vierte Quartal 2025 veröffentlicht und ein zweistelliges Umsatzwachstum... ► Artikel lesen |
| Plug Power: Plus 10 Prozent - darum hebt die Aktie ab | Neues Lebenszeichen bei Plug Power: Die Aktie zieht am Dienstag im vorbörslichen US-Handel um bis zu zehn Prozent an. Auslöser waren besser als erwartete Zahlen für das vierte Quartal. Besonders eine... ► Artikel lesen |
| Infosys and Intel Deepen Strategic Collaboration to Unlock AI Value for Enterprises Globally | Focus on scaling production-ready deployments, optimizing performance, and delivering measurable enterprise outcomes across industries with Infosys Topaz and Intel's... ► Artikel lesen |
| Ericsson And Intel Partner To Advance AI-Native 6G Networks | SANTA CLARA (dpa-AFX) - Telefonaktiebolaget LM Ericsson (ERIC) and Intel Corporation (INTC) said Monday they are expanding their partnership to accelerate the industry's shift from 6G research... ► Artikel lesen |
| ASML bringt nächste Generation von Chipmaschinen - Was bedeutet das für Intel und TSMC? | ASML hat die nächste Generation seiner Chipfertigungsmaschinen fertiggestellt. Diese hochentwickelten EUV-Werkzeuge, die 400 Millionen US-Dollar kosten, könnten die Chipproduktion für Unternehmen wie... ► Artikel lesen |
| Iran-Konflikt trifft Luxus: LVMH und Co unter Druck | Die militärische Eskalation im Nahen Osten hat die Luxusaktien aus ihrer leichten Erholungsphase gerissen. Denn gerade die angegriffene Golfregion ist einer der wichtigsten Märkte für LVMH und Co. An... ► Artikel lesen |
| LVMH: Ein klares Signal | Es ist ein historischer Meilenstein in der Unternehmensgeschichte: Die Gründerfamilie Arnault hält bei LVMH mehr als die Hälfte des Kapitals und sichert sich damit offiziell die absolute Mehrheit am... ► Artikel lesen |
| Familie Arnault hält über 50 Prozent an LVMH | DJ Familie Arnault hält über 50 Prozent an LVMH
Von Andrea Figueras
DOW JONES--Der französische Milliardär Bernard Arnault hat die Schwelle von 50 Prozent der Anteile an LVMH überschritten.... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu EUPRAXIA PHARMACEUTICALS INC finden Sie auf Wallstreet Online.