| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
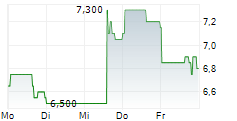
| 6,850 | 7,450 | 11:49 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 23.02. | Bloom Burton initiates Eupraxia stock with buy rating on EoE drug | 1 | Investing.com | ||
| EUPRAXIA PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| 21.02. | Eupraxia Pharmaceuticals Inc: Eupraxia Pharmaceuticals to list 6,428,574 more shares | 1 | Stockwatch | ||
| 21.02. | Eupraxia Pharmaceuticals Inc: Eupraxia Pharmaceuticals closes $63.2M (U.S.) offering | 1 | Stockwatch | ||
| 20.02. | Eupraxia-Aktie legt nach Kapitalerhöhung über 63 Mio. US-Dollar zu | 1 | Investing.com Deutsch | ||
| 20.02. | Eupraxia Pharmaceuticals closes $63.2M public offering | 1 | Investing.com | ||
| 20.02. | Eupraxia Pharmaceuticals Inc.: Eupraxia Pharmaceuticals Announces Closing of US$63.2 Million Public Offering Including Full Exercise of Underwriter Option | 166 | GlobeNewswire (Europe) | VICTORIA, British Columbia, Feb. 20, 2026 (GLOBE NEWSWIRE) -- Eupraxia Pharmaceuticals Inc. ("Eupraxia" or the "Company") (NASDAQ:EPRX) (TSX:EPRX), a clinical-stage biotechnology company leveraging... ► Artikel lesen | |
| 19.02. | Eupraxia Pharmaceuticals Inc: Eupraxia prices $55-million (U.S.) public offering | 1 | Stockwatch | ||
| 19.02. | Eupraxia Pharmaceuticals stock falls after pricing $55M public offering | 1 | Seeking Alpha | ||
| 19.02. | Eupraxia Pharmaceuticals prices $55 million public offering | 3 | Investing.com | ||
| 19.02. | Eupraxia Pharmaceuticals platziert öffentliches Angebot über 55 Millionen US-Dollar | 3 | Investing.com Deutsch | ||
| 19.02. | Eupraxia Pharmaceuticals Inc.: Eupraxia Pharmaceuticals Announces Pricing of US$55 Million Public Offering of Common Shares and Pre-Funded Warrants | 4 | GlobeNewswire (USA) | ||
| 18.02. | EUPRAXIA PHARMACEUTICALS INC. - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 18.02. | Eupraxia announces public offering of common shares | 2 | Investing.com | ||
| 18.02. | Eupraxia Pharmaceuticals Inc.: Eupraxia Pharmaceuticals Announces Proposed Public Offering | 625 | GlobeNewswire (Europe) | VICTORIA, British Columbia, Feb. 18, 2026 (GLOBE NEWSWIRE) -- Eupraxia Pharmaceuticals Inc. ("Eupraxia" or the "Company") (NASDAQ:EPRX) (TSX:EPRX), a clinical-stage biotechnology company leveraging... ► Artikel lesen | |
| 05.02. | EUPRAXIA PHARMACEUTICALS INC. - S-8, Securities to be offered to employees in employee benefit plans | - | SEC Filings | ||
| 15.01. | Cantor Fitzgerald raises Eupraxia Pharmaceuticals stock price target to $19 | 2 | Investing.com | ||
| 08.01. | Eupraxia Trial Data Shows Lasting Tissue Healing In Patients With Inflamed Esophagus | 1 | Benzinga.com | ||
| 08.01. | EUPRAXIA PHARMACEUTICALS INC. - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 08.01. | Eupraxia meldet positive Daten aus Studie zur Behandlung von EoE | 1 | Investing.com Deutsch | ||
| 08.01. | Eupraxia reports positive data from EoE treatment trial | 3 | Investing.com |
1 2 3 Weiter >>
57 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIOGEN | 158,85 | -1,27 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| INOVIO PHARMACEUTICALS | 1,450 | -2,68 % | INOVIO Pharmaceuticals, Inc.: FDA Accepts for Review INOVIO's BLA for INO-3107 for the Treatment of Adults with Recurrent Respiratory Papillomatosis (RRP) | PLYMOUTH MEETING, Pa., Dec. 29, 2025 /PRNewswire/ -- INOVIO (NASDAQ: INO), a biotechnology company focused on developing and commercializing DNA medicines to help... ► Artikel lesen | |
| VIKING THERAPEUTICS | 27,670 | -2,96 % | Viking Therapeutics: The High-Stakes Weight Loss Contender | ||
| TEMPUS AI | 43,200 | -5,26 % | Tempus AI Quartalszahlen: 90 % Umsatzplus bestätigen KI-Trend in der Medizin | CHICAGO, Illinois (IT-Times) - Das Diagnose-Technologie-Unternehmen Tempus AI hat seine Ergebnisse für das vierte Quartal 2025 veröffentlicht und ein rasantes Umsatzwachstum verzeichnet. Tempus AI -... ► Artikel lesen | |
| ABIVAX | 95,10 | -2,96 % | 2 Reasons Abivax Stock Could 10X by 2036 | ||
| PACIFIC BIOSCIENCES OF CALIFORNIA | 1,413 | +1,64 % | PACIFIC BIOSCIENCES OF CALIFORNIA, INC. - S-8, Securities to be offered to employees in employee benefit plans | ||
| EXACT SCIENCES | 88,83 | +0,49 % | Exact Sciences (EXAS) Merger with Abbott Moving Forward After Shareholder Approval | ||
| ARBUTUS BIOPHARMA | 4,004 | -0,10 % | Arbutus Biopharma Corporation: Arbutus Reports Third Quarter 2025 Financial Results and Provides Corporate Update | Strong financial position with cash, cash equivalents and marketable securities of $93.7M Moderna litigation U.S. trial scheduled for March 2026;Favorable claim construction ruling in Pfizer-BioNTech... ► Artikel lesen | |
| XOMA ROYALTY | 22,200 | -1,77 % | XOMA Royalty Corp - 8-K, Current Report | ||
| CARDIFF ONCOLOGY | 1,592 | -4,33 % | Cardiff Oncology, Inc.: Cardiff Oncology Reports Full Year 2025 Results and Provides Business Update | Reported positive update from Phase 2 CRDF-004 trial in first-line RAS-mutated mCRC, with the 30 mg onvansertib + FOLFIRI/bev arm demonstrating:• Robust ORR of 72.2% (vs 43.2% with combined SoC of... ► Artikel lesen | |
| IMMUNIC | 0,858 | -5,40 % | EQS-News: Immunic AG: Immunic, Inc. Reports Year-End 2025 Financial Results and Provides Corporate Update | Issuer: Immunic AG
/ Key word(s): Annual Results
Immunic, Inc. Reports Year-End 2025 Financial Results and Provides Corporate Update
26.02.2026 / 12:30 CET/CEST
The... ► Artikel lesen | |
| IOVANCE BIOTHERAPEUTICS | 3,023 | -6,88 % | Iovance Biotherapeutics, Inc.: Iovance Announces Positive Results from the First Clinical Trial for TIL Cell Therapy in Soft Tissue Sarcomas | 50% Objective Response Rate (ORR) in Advanced Sarcomas Significant Market Opportunity with More than 8,000 Patients Diagnosed Annually in the U.S. and Europe SAN CARLOS, Calif., Feb. 24, 2026 (GLOBE... ► Artikel lesen | |
| GUARDANT HEALTH | 77,72 | -1,52 % | Guardant Health, Inc. (GH) Strengthens Health Technology Portfolio with MetaSight Diagnostics Acquisition | ||
| CYTOKINETICS | 52,50 | -0,94 % | Cytokinetics, Incorporated: Cytokinetics Reports Fourth Quarter 2025 Financial Results and Provides Business Update | MYQORZO® Approved for Adults with Symptomatic Obstructive HCM in U.S., China and Europe; U.S. Launch Underway with First Prescriptions Dispensed within Days of Drug Availability Supplemental NDA for... ► Artikel lesen | |
| COHERUS ONCOLOGY | 1,358 | -4,10 % | Coherus Oncology, Inc.: Coherus Oncology to Report Full Year and Fourth Quarter 2025 Financial Results on March 9, 2026 |
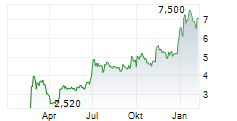
EUPRAXIA PHARMACEUTICALS INC gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell +0,74 % bei einem Aktienkurs von 6,850 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu EUPRAXIA PHARMACEUTICALS INC finden Sie auf Wallstreet Online.