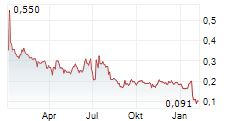
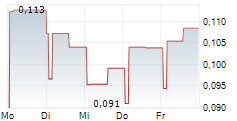
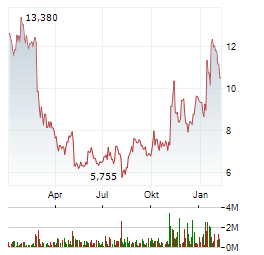
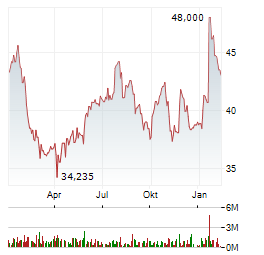
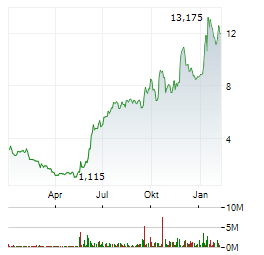
Chart-Typ:
Zeitraum:
| Servier; Day One Biopharmaceuticals: Servier and Day One Biopharmaceuticals announce acquisition to expand Servier's rare oncology portfolio | Acquisition positions Servier as a leader in pediatric low-grade glioma and expands its pipeline with programs targeting adult and pediatric cancers with high unmet needs. Transaction represents... ► Artikel lesen |
| Day One Biopharmaceuticals, Inc.: Servier and Day One Biopharmaceuticals announce acquisition to expand Servier's rare oncology portfolio | Acquisition positions Servier as a leader in pediatric low-grade glioma and expands its pipeline with programs targeting adult and pediatric cancers with high unmet needs Transaction represents total... ► Artikel lesen |
| Day One Biopharmaceuticals, Inc.: Day One Reports Fourth Quarter and Full Year 2025 Financial Results and Reaffirms 2026 Outlook and Revenue Guidance | OJEMDA 2025 momentum reflected by Q4 and full year net product revenues of $52.8 million and $155.4 million, respectively 2026 U.S. net product revenue projected at $225 - $250 million Expanded pipeline... ► Artikel lesen |
| PTA-NVR: QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG | DJ PTA-NVR: QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG
Gesamtstimmrechte gemäß -- 41 WpHG
QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach... ► Artikel lesen |
| Shortseller-Positionen aktuell: Auto1, Energiekontor, freenet, Gerresheimer, Puma, Qiagen, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| Erasca, Inc.: Erasca and Tango Therapeutics Enter into Clinical Collaboration to Evaluate Combination of ERAS-0015 and Vopimetostat | ERAS-0015, a pan-RAS molecular glue, will be evaluated in combination with PRMT5 inhibitor vopimetostat Tango will sponsor the clinical trial and Erasca will supply ERAS-0015 at no cost SAN DIEGO... ► Artikel lesen |
| Tango Therapeutics Sub, Inc: Tango Therapeutics Announces CEO Transition: Barbara Weber to Retire, Malte Peters Appointed Successor | Founding CEO Barbara Weber, M.D. to become executive chair of the board of directorsMalte Peters, M.D., current board member, appointed President and Chief Executive Officer, effective immediately... ► Artikel lesen |
| Tango Therapeutics Sub, Inc: Tango Therapeutics Reports Third Quarter 2025 Financial Results and Provides Business Highlights | - Data update from vopimetostat (TNG462) showed 2L MTAP-del pancreatic cancer median progression free survival (mPFS) 7.2 months- - Combination studies with RAS(ON) inhibitors ongoing, data anticipated... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu PILA PHARMA AB finden Sie auf Wallstreet Online.