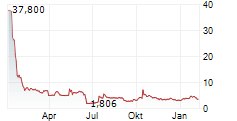
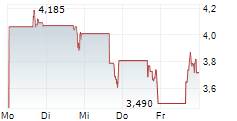
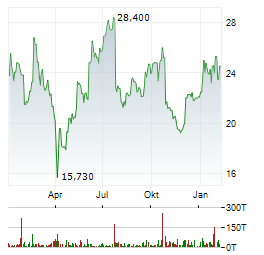
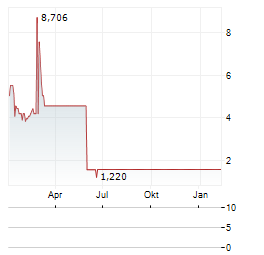
Chart-Typ:
Zeitraum:
| AKTIEN IM FOKUS: BE Semi wieder stark - Bofa aber skeptisch für Chip-Branche | FRANKFURT/AMSTERDAM (dpa-AFX) - Die Aktien von BE Semiconductor haben am Freitag positiv aus Europas Chipbranche herausgeragt. Sie zogen mit plus 5 Prozent wieder in Richtung Rekordhoch nach positiven... ► Artikel lesen |
| AKTIEN IM FOKUS: Starker Ausblick von Analog Devices treibt Infineon und STM | FRANKFURT/NEW YORK (dpa-AFX) - Die Aktien von Analog Devices sind am Mittwoch im vorbörslichen US-Handel um 8 Prozent auf ein Rekordhoch von fast 365 US-Dollar gestiegen. Ergebnisse und Ausblick des... ► Artikel lesen |
| STMICROELECTRONICS profitiert von einer erweiterten strategischen Partnerschaft mit Amazon | STMicro liefert in den kommenden Jahren verschiedene Halbleiter für AMAZONs Cloud-Sparte AWS. Bestandteil der Vereinbarung ist ein aktienbasiertes Element: STMicro begab AWS-Optionsscheine zum Kauf... ► Artikel lesen |
| Morning Market Movers: TechCreate, VivoSim Labs, La Rosa Holdings, Kaixin Holdings See Big Swings | BEIJING (dpa-AFX) - At 7:40 a.m. ET on Friday, premarket trading is seeing notable activity in several stocks, with early price movements signaling potential opportunities before the opening... ► Artikel lesen |
| VivoSim Labs, Inc.: VivoSim Labs Appoints Amar Sethi, M.D., Ph.D. as Chief Scientific Officer | SAN DIEGO, Jan. 06, 2026 (GLOBE NEWSWIRE) -- VivoSim Labs, Inc. (Nasdaq: VIVS) (the "Company" or "VivoSim Labs"), a pharmaceutical and biotechnology services company that is focused on providing testing... ► Artikel lesen |
| INOVIO Pharmaceuticals, Inc.: FDA Accepts for Review INOVIO's BLA for INO-3107 for the Treatment of Adults with Recurrent Respiratory Papillomatosis (RRP) | PLYMOUTH MEETING, Pa., Dec. 29, 2025 /PRNewswire/ -- INOVIO (NASDAQ: INO), a biotechnology company focused on developing and commercializing DNA medicines to help... ► Artikel lesen |
| INOVIO Pharmaceuticals, Inc.: INOVIO Reports Third Quarter 2025 Financial Results and Recent Business Highlights | Completed rolling Biologics License Application (BLA) submission seeking accelerated approval for lead candidate INO-3107; requested priority review
Expect... ► Artikel lesen |
| INOVIO Pharmaceuticals, Inc.: INOVIO Completes Rolling BLA Submission Seeking Accelerated Approval for INO-3107 as a Treatment for RRP in Adults | Recurrent respiratory papillomatosis (RRP) is a rare HPV-related disease of the respiratory tract with significant unmet need
INO-3107 previously received... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu STARDUST POWER INC finden Sie auf Wallstreet Online.