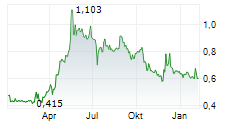
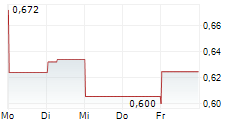
Cereno Scientific's second pipeline asset, CS014, continues to make steady progress through the clinic, with the company successfully completing the single ascending dose (SAD) part of the Phase I study which commenced in June 2024. The SAD part focused on evaluating the safety, tolerability and pharmacokinetics (PK) of CS014 in 30 healthy volunteers; results showcased a favourable safety profile. The second, multiple ascending dose (MAD) part of the study, which commenced in November 2024, is ongoing and management expects results in mid-2025, in line with previous guidance. CS014 is the company's second histone deacetylase inhibitor (HDACi) and has demonstrated anti-fibrotic, reverse remodelling and anti-thrombotic properties in preclinical studies. The target indication for CS014 is idiopathic pulmonary fibrosis (IPF), a rare, progressive disease, with no curative treatments and an average survival of three to five years. Management intends to commence Phase II in H126.Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research