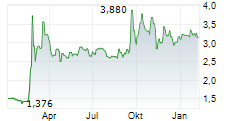
Oryzon has reported its FY24 results, reflecting steady progress across its pipeline. Oryzon's highest value-driving programme remains vafidemstat in borderline personality disorder (BPD), which, following a positive end-of-Phase II (EoP2) meeting in October, is now Phase III-ready. As the company transitions into a Phase III organisation, it is reshaping its board of directors to enhance its US outreach, which we believe is a sensible strategy given that the US will be the key market for Oryzon's clinical candidates. In oncology, Oryzon continues its broad approach to bolster its data package for iadademstat in acute myeloid leukaemia (AML). FRIDA remains the top priority and the next update is expected in December 2025. Following the FY24 results, our valuation for Oryzon adjusts to €885.1m or €13.5/share (from €796m or €12.3/share previously).Den vollständigen Artikel lesen ...
© 2025 Edison Investment Research