| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 2,690 | 2,760 | 08:42 | |
| 2,690 | 2,755 | 08:40 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Oryzon Genomics SA FullYear Loss Widens | - | RTTNews | ||
| Fr | Oryzon Genomics, S.A.: ORYZON Reports Financial Results and Corporate Update for Quarter Ended December 31st, 2025 | 481 | GlobeNewswire (Europe) | Strong cash position at year-end 2025: $33.3 million (€28.4 million) CNS(Vafidemstat) Appointed renowned Rolando Gutierrez-Esteinou, M.D. as new CMO for CNSPreparing protocol resubmission to incorporate... ► Artikel lesen | |
| ORYZON GENOMICS Aktie jetzt für 0€ handeln | |||||
| 25.02. | EMA approves Oryzon Genomics' application to initiate Phase II study with iadademstat | 2 | thecorner.eu | ||
| 03.02. | Oryzon obtains grant from Japanese Patent Office for vafidemstat | 2 | thecorner.eu | ||
| 23.12.25 | Oryzon Genomics obtains patent in Japan for 'Combinations of iadademstat for cancer therapy' | 1 | thecorner.eu | ||
| 22.12.25 | Oryzon Genomics, S.A.: ORYZON Expands Global Patent Protection for Iadademstat with Grant Decision in Japan Covering Combinations with PD-1/PD-L1 Inhibitors | 341 | GlobeNewswire (Europe) | MADRID and CAMBRIDGE, Mass., Dec. 22, 2025 (GLOBE NEWSWIRE) -- Oryzon Genomics, S.A. (ISIN Code: ES0167733015, ORY), a clinical-stage biopharmaceutical company and global leader in epigenetics, today... ► Artikel lesen | |
| 15.12.25 | Oryzon to increase capital through cash contributions and with exclusion of pre-emptive subscription rights, for effective amount of up to €125 million | 2 | thecorner.eu | ||
| 15.12.25 | Oryzon Genomics, S.A.: ORYZON Announces the Voting Results of December 2025 Extraordinary General Shareholders' Meeting | 342 | GlobeNewswire (Europe) | 37.8% of voting rights present or representedAll resolutions were approved MADRID and CAMBRIDGE, Mass., Dec. 15, 2025 (GLOBE NEWSWIRE) -- Oryzon Genomics S.A. (ISIN Code: ES0167733015, ORY), a clinical-stage... ► Artikel lesen | |
| 12.12.25 | ORYZON GENOMICS, S.A.: ORYZON announces the voting results from its Extraordinary General Shareholders' Meeting | 1 | CNMV | ||
| 09.12.25 | Oryzon Genomics, S.A.: ORYZON Presents Data for Iadademstat Combinations in AML at the American Society of Hematology (ASH) 67th Annual Meeting | 1 | GlobeNewswire (USA) | ||
| 09.12.25 | ORYZON GENOMICS, S.A.: ORYZON presents encouraging updated data for iadademstat in AML at the American Society of Hematology (ASH) Meeting | 3 | CNMV | ||
| 11.11.25 | Oryzon Genomics - Progression across the pipeline in Q3 | 360 | Edison Investment Research | Oryzon Genomics has reported its Q325 results, a period characterised by tangible progress across its ongoing clinical programmes. Anticipation builds for vafidemstat in borderline personality disorder... ► Artikel lesen | |
| 10.11.25 | ORYZON GENOMICS, S.A.: Call of the Extraordinary General Shareholders' Meeting of Oryzon Genomics, S.A. | 3 | CNMV | ||
| 07.11.25 | Oryzon Genomics S.A. GAAP EPS of $0.01 | 1 | Seeking Alpha | ||
| 07.11.25 | Oryzon Genomics, S.A.: ORYZON Reports Financial Results and Corporate Update for Quarter Ended September 30, 2025 | 225 | GlobeNewswire (Europe) | The net result as of September 30, 2025 improved by $1.1M compared to September 2024, following termination of a Convertible Bonds financing facility with Nice&Green CNS(Vafidemstat) Received feedback... ► Artikel lesen | |
| 04.11.25 | Oryzon Genomics - Progress for iadademstat across the board | 311 | Edison Investment Research | Oryzon has announced encouraging updates for iadademstat. In acute myeloid leukaemia (AML), positive data was reported for two programmes, including the lead oncology programme, FRIDA. Updated interim... ► Artikel lesen | |
| 03.11.25 | Oryzon Genomics, S.A.: ORYZON Announces First-Patient-In (FPI) in RESTORE Phase Ib Trial of Iadademstat in Sickle Cell Disease | 246 | GlobeNewswire (Europe) | Recently approved by the European Medicines Agency, RESTORE will evaluate iadademstat's ability to raise fetal hemoglobin (HbF) in adult patients with SCDIadademstat has demonstrated a favorable safety... ► Artikel lesen | |
| 30.10.25 | Oryzon Genomics, S.A.: ORYZON to Participate in Upcoming Events in November | 3 | GlobeNewswire (USA) | ||
| 20.10.25 | Oryzon Genomics - Making headway towards Phase III in BPD | 380 | Edison Investment Research | Oryzon Genomics has announced that it has received FDA feedback on its plans for the Phase III programme in borderline personality disorder (BPD) for its lead central nervous system (CNS) drug candidate... ► Artikel lesen | |
| 17.10.25 | Oryzon Genomics: FDA gibt Feedback zu Phase-III-Studienprotokoll für Vafidemstat | 2 | Investing.com Deutsch |
1 2 Weiter >>
39 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 87,25 | +0,58 % | BioNTech Aktie: Die Wende naht! Golden Cross! | © Foto: 2026 BioNTech SE. Alle Recht vorbehalten.Fast wie in der Serie "Game of Thrones", wo der Winter naht, könnte bei BioNTech die Wende nahen. Nach dem Kursrausch in der Corona-Ära folgte der brutale... ► Artikel lesen | |
| EVOTEC | 5,304 | 0,00 % | Evotec Aktie vor dem Durchbruch? Diesmal ist etwas anders... | Die Evotec Aktie hat zuletzt ein wichtiges Muster bestätigt: Wiederholt scheiterte die Biotech-Aktie aus Hamburg mit Breakversuchen an der 200-Tage-Linie. Nach mehr oder weniger starken Ausbrüchen hierüber... ► Artikel lesen | |
| MEDIGENE | 0,039 | 0,00 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 40,700 | 0,00 % | Shortseller-Positionen aktuell: freenet, Gerresheimer, Hypoport, Qiagen, Renk, Suss MicroTec, TeamViewer | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 45,000 | +4,91 % | Moderna: Kombi-Impfung für Grippe und Covid erhält Zulassung | Gute Nachrichten für Moderna: Die EU-Arzneimittelbehörde EMA hat grünes Licht gegeben für den ersten Kombi-Impfstoff gegen Corona und Grippe. Der Wirkstoff solle für Menschen ab 50 Jahren zugelassen... ► Artikel lesen | |
| PAION | 0,060 | -35,21 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WURDE STATTGEGEBEN | Dem Mistrade-Antrag wurde stattgegeben. Folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wurden aufgehoben:ISIN Datum Zeit Volumen PreisDE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 4,428 | -0,05 % | Original-Research: Valneva (von First Berlin Equity Research GmbH): Buy | Original-Research: Valneva - von First Berlin Equity Research GmbH
25.02.2026 / 17:11 CET/CEST
Veröffentlichung einer Research, übermittelt durch EQS News - ein Service der EQS Group.
Für den Inhalt... ► Artikel lesen | |
| AMGEN | 322,50 | -0,65 % | Amgen EVP Sold $20.77M In Company Stock | ||
| EPIGENOMICS | 1,000 | +4,17 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 8,111 | -0,76 % | FOMO an der Wall Street: Novavax meldet überraschenden Gewinn - Aktie schießt zweistellig hoch | © Foto: picture alliance / Ulrich Baumgarten | Ulrich BaumgartenEin Ergebnis, das niemand erwartet hatte, gepaart mit neuen Big-Pharma-Deals: Novavax sorgt für FOMO an der Wall Street. Wie lange hält... ► Artikel lesen | |
| STRYKER | 330,70 | -0,12 % | Stryker highlights new additions to Mako, Triathlon portfolios at AAOS | ||
| BIOGEN | 157,65 | 0,00 % | WONBIOGEN Co., Ltd: Won BioGen Accelerates Global Market Expansion with Advanced Moist Wound Care Technology | SEOUL, South Korea, Feb. 24, 2026 /PRNewswire/ -- Medical device manufacturer Won BioGen (CEO Kim Won-il) is accelerating its global market expansion based on its advanced moist wound care... ► Artikel lesen | |
| BIOFRONTERA | 2,770 | +2,97 % | Paradigmenwechsel in der onkologischen Dermatologie: Vidac Pharma als Innovator, was machen Almirall und Biofrontera? | Die onkologische Dermatologie steht an der Schwelle zu einer Revolution, die das bisherige Verständnis der Krebsbehandlung grundlegend verändern könnte. Der globale Markt für die Behandlung der Aktinischen... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,860 | +0,70 % | HEIDELBERG PHARMA AG unter Beobachtung | ||
| ILLUMINA | 110,04 | -1,34 % | Evercore ISI reiterates Illumina stock rating on competitive positioning |
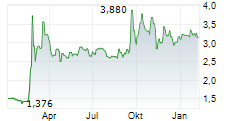
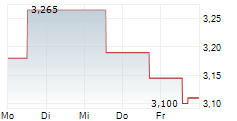
ORYZON GENOMICS SA gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 2,740 Euro und damit +0,18 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ORYZON GENOMICS SA finden Sie auf Wallstreet Online.